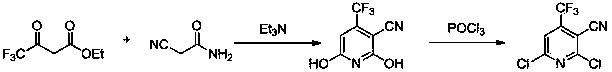
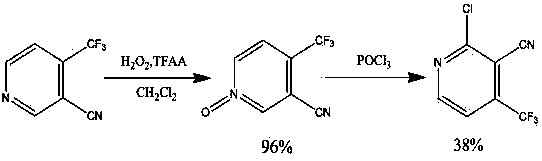
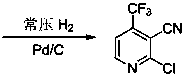Preparation method for 2-chlorine-4-trifluoromethyl-3-cyanopyridine
A technology of trifluoromethyl and cyanopyridine, which is applied in the chemical field, can solve the problems of complex operation, low product yield, and high cost, and achieve the effects of low reaction cost, high yield, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method of 2-chloro-4-trifluoromethyl-3-cyanopyridine, comprising the steps of:
[0031] (1) Synthesis of 4,6-dihydroxy-4-trifluoromethyl-3-cyanopyridine (Ⅲ)
[0032] Add 80g of ethyl trifluoroacetoacetate to the reactor, then add 36.5g of cyanoacetamide, then dissolve it in 400g of 1,4-dioxane, add 44g of triethylamine, heat to 75°C and reflux for 15 hours, It was detected by thin-layer chromatography that the reaction was complete, spin-dried, added 30 g of ether, stirred for 15 minutes, a white solid precipitated, filtered, and the filter cake was dried to obtain a white solid 4,6-dihydroxy-4-trifluoromethyl-3-cyanide Base pyridine 77.6g, productive rate is 87.5%.
[0033] (2) Synthesis of 2,6-dichloro-4-trifluoromethyl-3-cyanopyridine (Ⅱ)
[0034] Add 90 g of the obtained 4,6-dihydroxy-4-trifluoromethyl-3-cyanopyridine into the reactor, add 48.3 g of tetramethylammonium chloride, 600 g of phosphorus oxychloride, and raise the temperature to 100 ° C. ...
Embodiment 2
[0041] (1) Synthesis of 4,6-dihydroxy-4-trifluoromethyl-3-cyanopyridine (Ⅲ)
[0042] Add 80g of ethyl trifluoroacetoacetate to the reactor, then add 65.8g of cyanoacetamide, then dissolve in 400g of ethanol, then add 80g of triethylamine, heat to 90°C and reflux for 12 hours, and the reaction is complete by thin layer chromatography , spin dry, add ether 50g, stir for 20 minutes, a white solid precipitates out, filter, and dry the filter cake to obtain 79.02g of white solid 4,6-dihydroxy-4-trifluoromethyl-3-cyanopyridine, the yield It was 89.1%.
[0043] (2) Synthesis of 2,6-dichloro-4-trifluoromethyl-3-cyanopyridine (Ⅱ)
[0044] Add 90 g of the obtained 4,6-dihydroxy-4-trifluoromethyl-3-cyanopyridine into the reactor, add 62.4 g of tetramethylammonium chloride and 800 g of phosphorus oxychloride, and raise the temperature to 80°C. React for 120 minutes, the reaction is complete by thin-layer chromatography, cool to room temperature, spin dry phosphorus oxychloride with a dr...
Embodiment 3
[0051] (1) Synthesis of 4,6-dihydroxy-4-trifluoromethyl-3-cyanopyridine (Ⅲ)
[0052] Add 80g of ethyl trifluoroacetoacetate to the reactor, then add 91.3g of cyanoacetamide, then dissolve it in 500g of isopropanol, then add 110g of triethylamine, heat to 80°C and reflux for 13 hours, and detect by thin layer chromatography After the reaction was complete, spin dry, add 50 g of diethyl ether, stir for 20 minutes, a white solid precipitated, filtered, and dried the filter cake to obtain 80.18 g of white solid 4,6-dihydroxy-4-trifluoromethyl-3-cyanopyridine, The yield was 90.4%.
[0053] (2) Synthesis of 2,6-dichloro-4-trifluoromethyl-3-cyanopyridine (Ⅱ)
[0054] Add 90 g of the obtained 4,6-dihydroxy-4-trifluoromethyl-3-cyanopyridine into the reactor, add 96.7 g of tetramethylammonium chloride and 900 g of phosphorus oxychloride, and raise the temperature to 50 ° C. Reacted for 150 minutes, the reaction was complete as detected by thin layer chromatography, cooled to room temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com