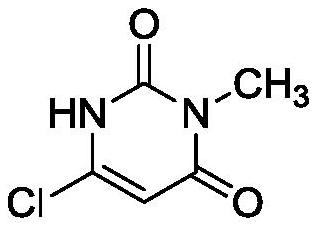
The preparation method of 6-chloro-3-methyluracil
A technology of methyl uracil and methyl urea, applied in the field of pharmaceutical chemical synthesis, can solve the problems of increasing production cost, low yield and the like, and achieves the effects of reducing usage, improving yield and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
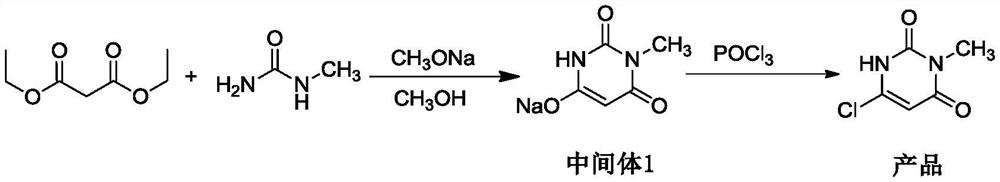
[0028] The present embodiment provides a kind of preparation method of 6-chloro-3-methyluracil, specifically, comprises the following steps:
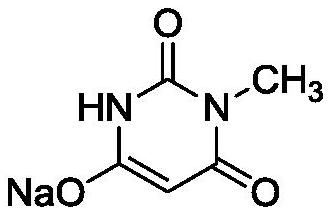
[0029] Step 1: Add 20.0g of methylurea, 110g of methanol and 102g of sodium methoxide into a 500mL four-neck flask, stir to dissolve, then add 47.6g of diethyl malonate, heat up and reflux for 6 hours, then cool to 20°C, add acetic acid Adjust the pH value of the reaction solution to 5.0, add 30mL of water, keep the reaction solution at 20°C for 15 minutes, cool the reaction solution to 10°C, and keep the temperature for 15 minutes, filter with suction, and dry to obtain 40.8g of white to off-white loose powder -like intermediate 1, the yield is 92.1%;
[0030] Step 2: Take 20.0g of intermediate 1 and add it to a 250mL four-necked bottle, then add 80g of acetonitrile and 2.5g of water in sequence, and then add 50.0g of phosphorus oxychloride dropwise, control the temperature at 10°C during the dropwise addition, and complete the dropwis...
Embodiment 2
[0034] The present embodiment provides a kind of preparation method of 6-chloro-3-methyluracil, specifically, comprises the following steps:
[0035] Step 1: Add 20.0g of methylurea, 110g of methanol and 117g of sodium methoxide into a 500mL four-neck flask, stir to dissolve, then add 51.9g of diethyl malonate, heat up and reflux for 10 hours, then cool to 30°C, add acetic acid Adjust the pH value of the reaction solution to 8.0, add 30mL of water, keep the reaction solution at 30°C for 45 minutes, cool the reaction solution to 12°C, and keep the temperature for 30 minutes, filter with suction, and dry to obtain 41.0g of white to off-white loose powder -like intermediate 1, the yield is 92.5%;
[0036] Step 2: Take 20.0g of intermediate 1 and add it to a 250mL four-necked bottle, then add 80g of acetonitrile and 2.5g of water in sequence, and then add 50.0g of phosphorus oxychloride dropwise, control the temperature at 5°C during the dropwise addition, and complete the dropwis...
Embodiment 3
[0040] The present embodiment provides a kind of preparation method of 6-chloro-3-methyluracil, specifically, comprises the following steps:
[0041] Step 1: Add 20.0g of methylurea, 110g of methanol and 110g of sodium methoxide into a 500mL four-neck flask, stir to dissolve, then add 49.7g of diethyl malonate, heat up and reflux for 8 hours, then cool to 25°C, add acetic acid Adjust the pH value of the reaction solution to 6.5, add 30mL of water, keep the reaction solution at 25°C for 30 minutes, cool the reaction solution to 11°C, and keep the temperature for 25 minutes, filter with suction, and dry to obtain 41.3g of white to off-white loose powder -like intermediate 1 with a yield of 93.2%;
[0042] Step 2: Take 20.0g of intermediate 1 and add it to a 250mL four-necked bottle, then add 80g of acetonitrile and 2.5g of water in sequence, and then add 50.0g of phosphorus oxychloride dropwise, control the temperature at 8°C during the dropwise addition, and complete the dropwi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com