Novel medicinal salt for cinepazide and preparation method thereof
A medicinal salt and pharmacy technology, applied in the field of medicine, can solve problems such as hidden dangers of clinical drug safety, inconvenient drug production, storage, transportation and use, and light sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
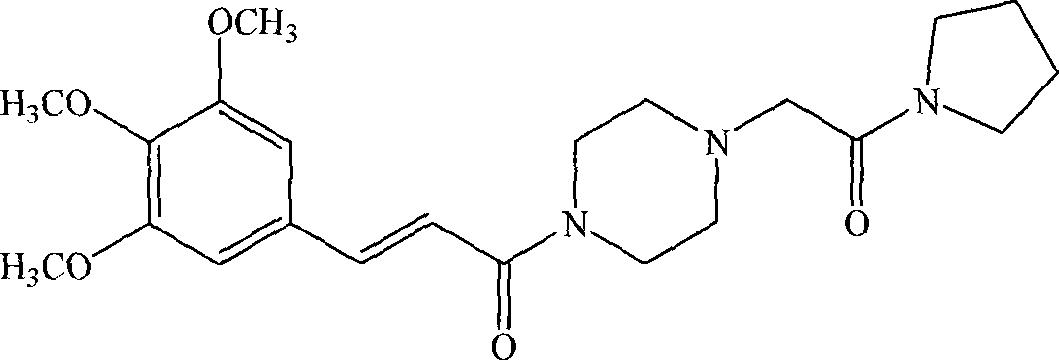
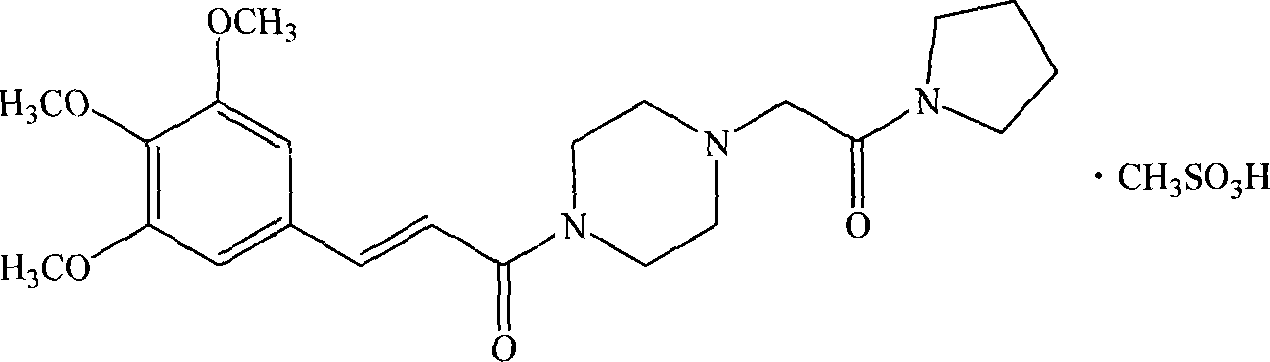
[0101] The preparation of embodiment 1 cinepazide mesylate
[0102] Take cinepazide 10g (24mmol), put it into the reaction bottle, add 50ml ethanol, stir to dissolve, then add dropwise 2.9g methanesulfonic acid (25mmol) / 50ml ethanol under stirring, let stand for 1h after the dropwise addition, the reaction solution Freeze and crystallize to obtain the off-white cinepazide mesylate crude product; add the crude product to 90ml of ethanol, stir and heat to reflux, and after the solid is completely dissolved, the solution is cooled and crystallized to obtain 9.5 g of white crystalline powder, yield: 77.2%.
[0103] 1 H-NMR (600MHz, DMSO) δ: 1.94[m, 4H], 2.33[s, 4H], 3.21[brs, 4H], 3.39[t, 4H], 3.56[brs, 2H], 3.69[s, 9H] ], 4.26[s, 2H], 4.50[brs, 2H], 7.08[s, 2H], 7.22[d, 1H], 7.52[d, 1H], 10.17[brs, 1H]
[0104] IR(KBr)cm -1 : 3442, 2951, 2868, 2585, 1657, 1448, 1425, 1271, 1232, 1035, 765, 557, 531
[0105] Elemental analysis (C 22 h 31 N 3 o 5 ·CH 3 SO 3 h)
[0106] ...
Embodiment 2
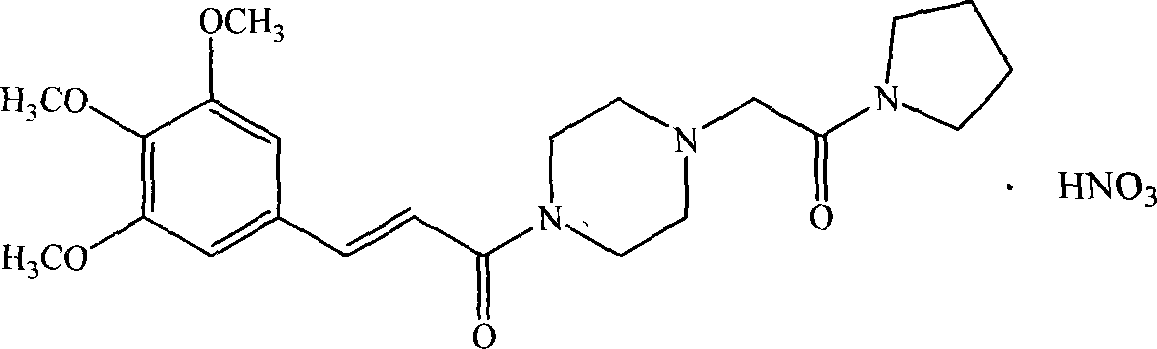
[0108] The preparation of embodiment 2 cinepazide nitrate
[0109] Take cinepazide 15g (36mmol), put it into the reaction bottle, then add 60ml of 95% ethanol, stir to dissolve, then add 2.4g of nitric acid (about 36mmol) / 10ml of ethanol dropwise under stirring, let stand for 1h after the dropwise addition, and react Liquid freezing and crystallization, the off-white cinepazide hydrochloride crude product was obtained; the crude product was added to 80ml ethanol / 15ml water, stirred and heated to reflux, after the solid was completely dissolved, the solution was cooled and crystallized to obtain 14.8g of white crystalline powder, the yield : 85.4%.
[0110] 1 H-NMR (600MHz, DMSO) δ: 2.01[m, 4H], 3.29[brs, 4H], 3.31[t, 4H], 3.61[brs, 2H], 3.67[s, 9H], 4.18[s, 2H] ], 4.55 [brs, 2H], 7.04 [s, 2H], 7.35 [d, 1H], 7.47 [d, 1H], 10.21 [brs, 1H]
[0111] IR(KBr)cm -1 : 3427, 2942, 2940, 2845, 1633, 1597, 1530, 1436, 1222, 1069, 847, 565, 528
[0112] Elemental analysis (C 22 h 3...
Embodiment 3
[0115] The preparation of embodiment 3 cinepazide phosphate
[0116] Take cinepazide 15g (36mmol), put it into the reaction bottle, then add 80ml of ethanol, stir to dissolve, then add dropwise 4.2g of phosphoric acid (36mmol) / 10ml of ethanol under stirring, stir for 1h after the dropwise addition, and let stand for 1h, The reaction solution was frozen and crystallized to obtain the crude product of off-white cinepazide phosphate; the crude product was added to 70ml of ethanol / 10ml of water, stirred and heated to reflux, and the solution was cooled and crystallized after the solid was completely dissolved to obtain 15.7g of white crystalline powder. Rate: 84.8%.
[0117] 1 H-NMR (600MHz, DMSO) δ: 2.04[m, 4H], 3.11[brs, 4H], 3.26[t, 4H], 3.49[brs, 2H], 3.78[s, 9H], 4.22[s, 2H] ], 4.60 [brs, 2H], 7.05 [s, 2H], 7.42 [d, 1H], 7.55 [d, 1H], 10.21 [brs, 3H]
[0118] IR(KBr)cm -1 : 3436, 2947, 2938, 2845, 1631, 1609, 1515, 1436, 1227, 1068, 847, 562, 527
[0119] Elemental analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com