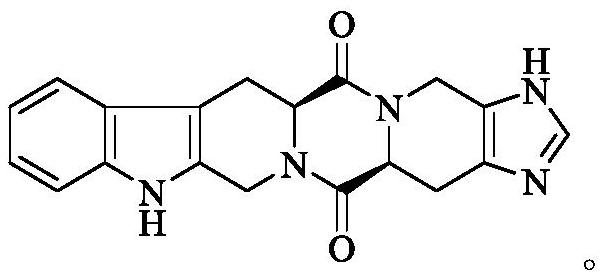
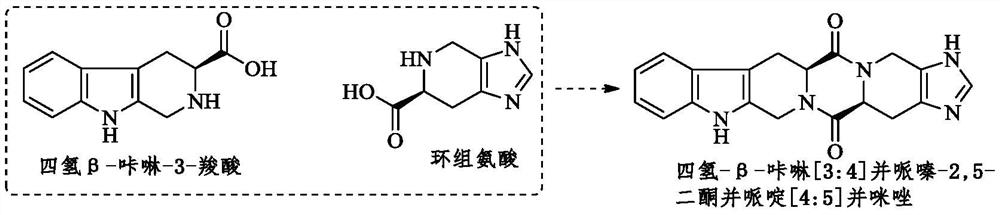
Hexacyclic piperazinedione compound and preparation, biological activity and application thereof
A piperazine, diketone technology, applied in the fields of anti-tumor growth, anti-tumor cell migration and invasion, and anti-tumor metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
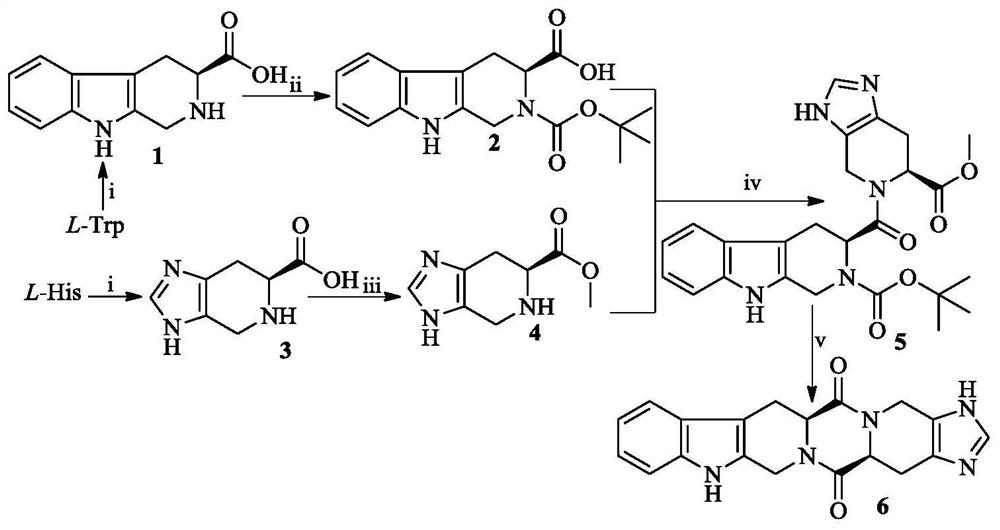
[0019]Example 1 Preparation of 3S-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (1)
[0020]Add 0.1 mL of concentrated sulfuric acid (98%) to 200 mL of distilled water at 0°C and stirring, then add 2.04 g (10.0 mmol) of L-Trp and stir until the solid is dissolved, and finally add 5 mL of aqueous formaldehyde solution (37%) and stir at room temperature for 6 hours. TLC showed that L-Trp disappeared completely. Ammonia solution (25%) was added to the reaction mixture at 0°C while stirring to adjust the pH to 7. The reaction mixture was allowed to stand at room temperature for 30 min, and the resulting precipitate was collected by filtration to obtain 2.03 g (93%) of the title compound as a pale yellow solid. ESI-MS(m / e):217[M+H]+;1H NMR(300MHz, DMSO-d6): δ / ppm=12.141(s,1H), 10.944(s,1H),7.436(s,1H),7.321(s,1H),7.032(d,2H), 4.207(m,3H), 2.822( m,2H).
Embodiment 2
[0021]Example 2 Preparation of 3S-2-tert-butoxycarbonyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (2)
[0022]To 0.972 g (4.5 mmol) of 3S-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (1), 10 mL of N,N-dimethylformamide was added, and the solid was suspended by stirring. Add 1.275g (1.3mmol) (Boc) to the suspension at 0℃ and stirring2O, then add triethylamine to adjust the pH to 10. The resulting solution was stirred at room temperature until TLC showed that compound 1 disappeared completely. The reaction mixture was concentrated under reduced pressure, and the obtained pale yellow oil was dissolved in 40 mL of ethyl acetate. The obtained ethyl acetate solution was washed with 5% potassium hydrogen sulfate aqueous solution (50 mL×3) and then with saturated sodium chloride aqueous solution (50 mL×3). 3), the ethyl acetate layer was dried with anhydrous sodium sulfate for 12h. After filtration, the filtrate was concentrated under reduced pressure, and the obtained light yellow ...
Embodiment 3
[0023]Example 3 Preparation of 6S-4,5,6,7-tetrahydro-imidazole[4:5]piperidine-6-carboxylic acid (3)
[0024]0.4 mL of concentrated sulfuric acid (98%) was added to a solution of 2.50 g (16.1 mmol) of L-His and 10 mL of distilled water at 0° C. and stirring to gradually dissolve L-His. Add 3 mL of aqueous formaldehyde solution (37%) to the resulting solution and heat it at 60°C for 6 h. TLC showed that L-His disappeared completely. The reaction mixture was stirred at 0°C, and then 25% aqueous ammonia solution was added to adjust the pH to 7. Let stand for 30min at room temperature and filter. The collected solid was washed three times with water and acetone each to obtain 2.649 g (98%) of the title compound as a colorless solid. ESI-MS(m / e):168[M+H]+;1H NMR(300MHz, DMSO-d6): δ / ppm=12.016(s, 1H), 7.548(s, 1H), 5.040(d, J=13.8Hz, 1H), 4.789(d, J=14.1Hz, 1H), 4.277(m, 1H) , 3.761(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com