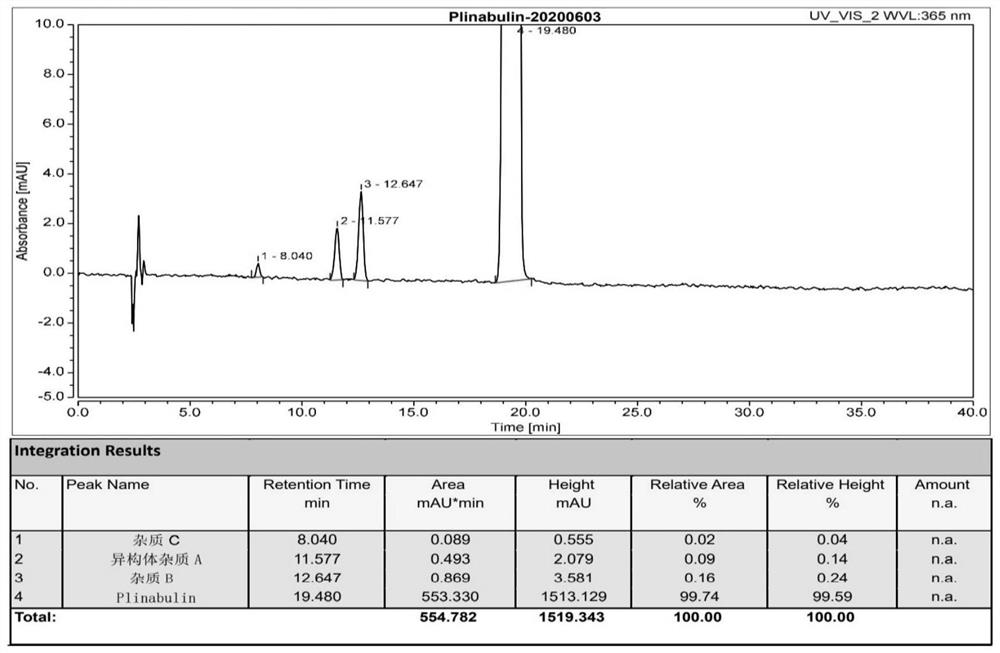
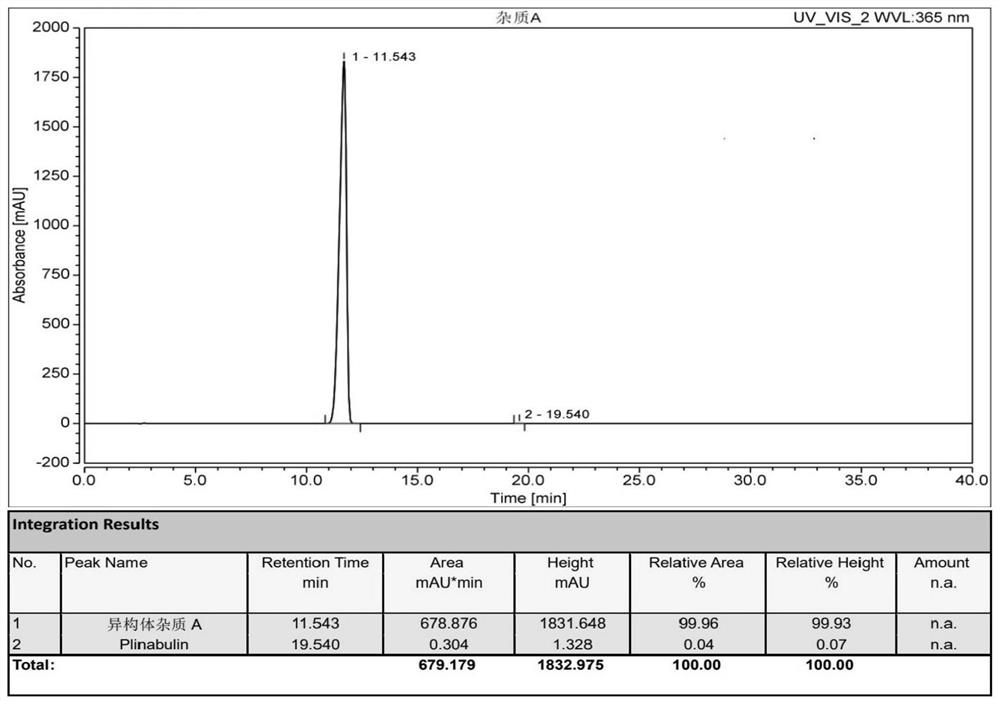
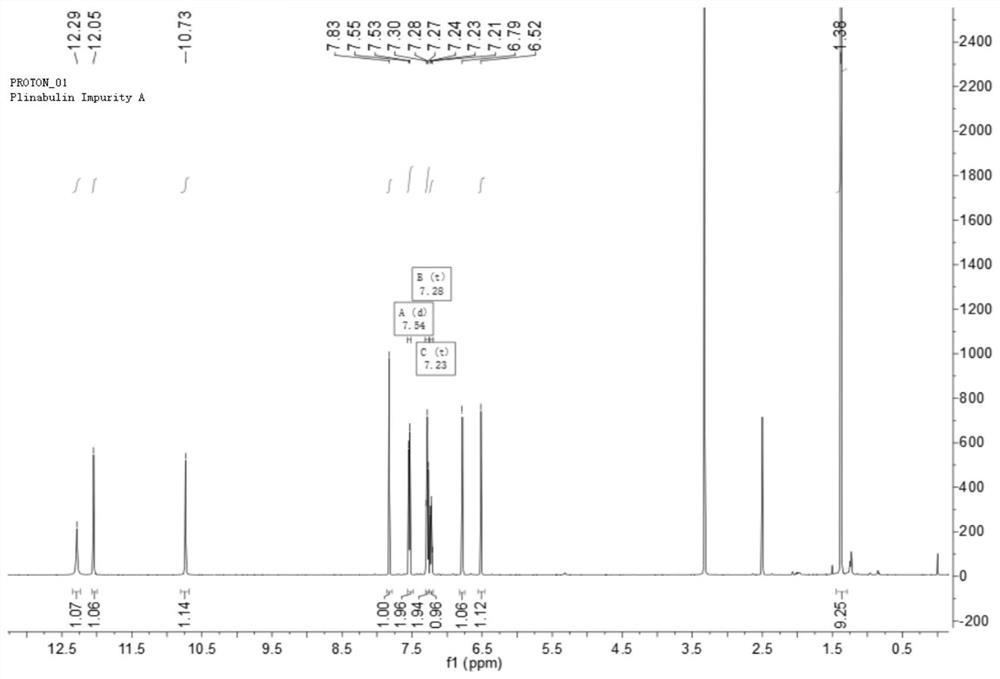
Preparation method and application of tubulin inhibitor plinabulin isomer impurity
A technology of Plinabulin and isomers, applied in the field of medicinal chemistry, can solve problems affecting product purity, etc., and achieve the effects of avoiding solvent loss, high yield, and simple operation of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of (3Z,6Z)-3-[(5-tert-butyl-1H-imidazol-4-yl)methylene]-6-(benzylidene)-2,5-piperazinedione
[0029]
[0030] Under nitrogen protection, add 5.0g (32.85mmol) 5-(tert-butyl)-1H-imidazole-4-carbaldehyde and 35mL DMF into the reaction flask, then add 13.02g (65.7mmol) N,N-diacetylpiperazine -2,5-diketone and 16.05g (49.28mmol) cesium carbonate were stirred and reacted at room temperature for 20h in the dark. The reaction solution was poured into (500 mL) ice water, and the suction filter cake was washed with water (100 mL*2) and petroleum ether / ethyl acetate 8 / 1 (400 mL) successively. The filter cake was dispersed ultrasonically with ethanol and dichloromethane, and filtered off. The insoluble matter was concentrated under reduced pressure, and anhydrous ethanol was added with water. Ethyl acetate (200mL) was beaten to give a brownish yellow solid (Z)-1-acetyl-3-((5-(tert-butyl)-1H-imidazol-4-yl)methylene)piperazine-2, 4.16 g of 5-diketone, yield 43.62%. ...
Embodiment 2
[0044] Preparation of (3Z,6E)-3-[(5-tert-butyl-1H-imidazol-4-yl)methylene]-6-(benzylidene)-2,5-piperazinedione
[0045]
[0046] In a 500mL glass reaction bottle, add (3Z,6Z)-3-[(5-tert-butyl-1H-imidazol-4-yl)methylene]-6-(phenylene Methyl)-2,5-piperazinedione 1.0g and 100mL tetrahydrofuran, stirring and dissolving at room temperature, stirring for 72 hours under the irradiation of 365nm ultraviolet lamp (300W), sampling HPLC monitoring during the reaction, until (3Z,6Z) -3-[(5-tert-butyl-1H-imidazol-4-yl) methylene]-6-(benzylidene)-2,5-piperazinedione remaining 45% when the reaction reached equilibrium, the The reaction solution was concentrated to dryness under reduced pressure to obtain 1.03 g of a yellow solid. Crystallization was attempted with various solvents on the solid. Some solvents are not easy to crystallize because of their better solubility, such as DMF, DMA, DMSO, etc.; some solvents are not conducive to crystallization because of their poor solubility, su...
Embodiment 3
[0051] Preparation of (3Z,6E)-3-[(5-tert-butyl-1H-imidazol-4-yl)methylene]-6-(benzylidene)-2,5-piperazinedione
[0052] Weigh the purity of 99.74% (3Z,6Z)-3-[(5-tert-butyl-1H-imidazol-4-yl)methylene]-6-(benzylidene)-2,5-piperazine Add 300mg of diketone into a 100mL glass reaction bottle, add 50mL of acetonitrile, stir and dissolve at room temperature, and stir for 72 hours under the irradiation of a 365nm ultraviolet lamp (300W) in a black airtight environment, and take samples to monitor the reaction by HPLC, (3Z, 6Z) -3-[(5-tert-butyl-1H-imidazol-4-yl)methylene]-6-(benzylidene)-2,5-piperazinedione remaining 43%, depressurize the reaction solution Concentrate to dryness to obtain a yellow solid, dissolve the yellow solid in 100mL of acetonitrile under reflux, add 50mL of purified water dropwise at the temperature, keep for 10min after the dropwise addition, naturally cool down to room temperature, stir and crystallize for 3 hours, a light yellow solid precipitates, filter and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com