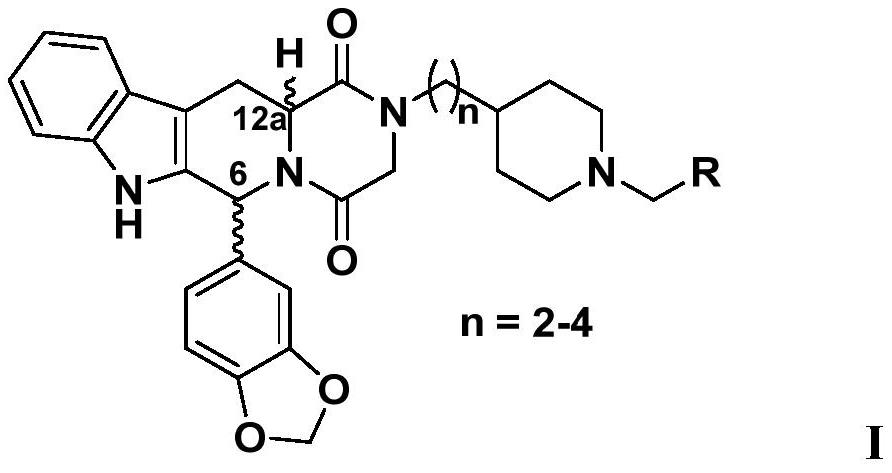
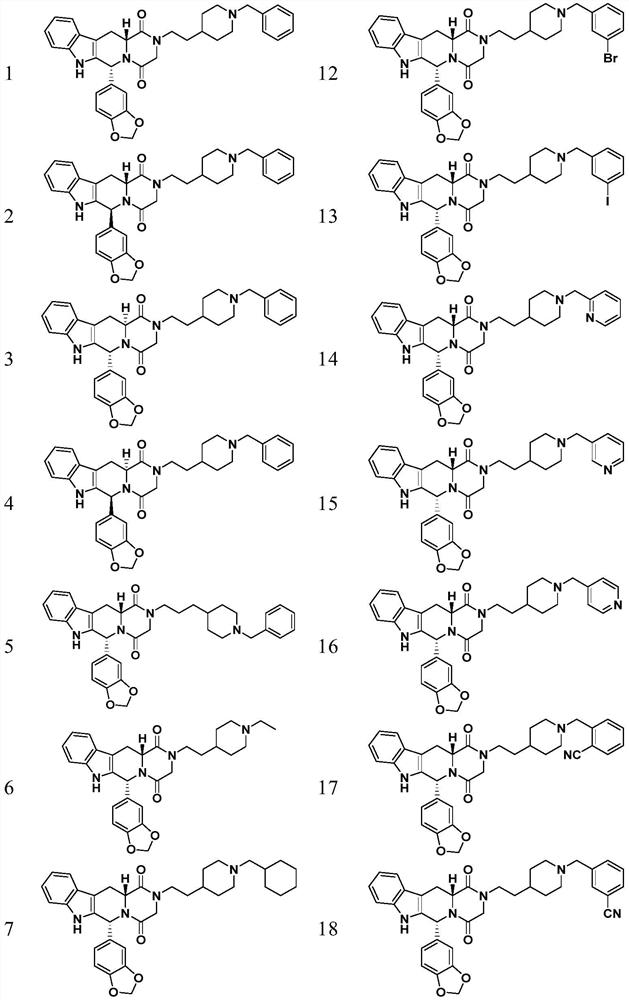
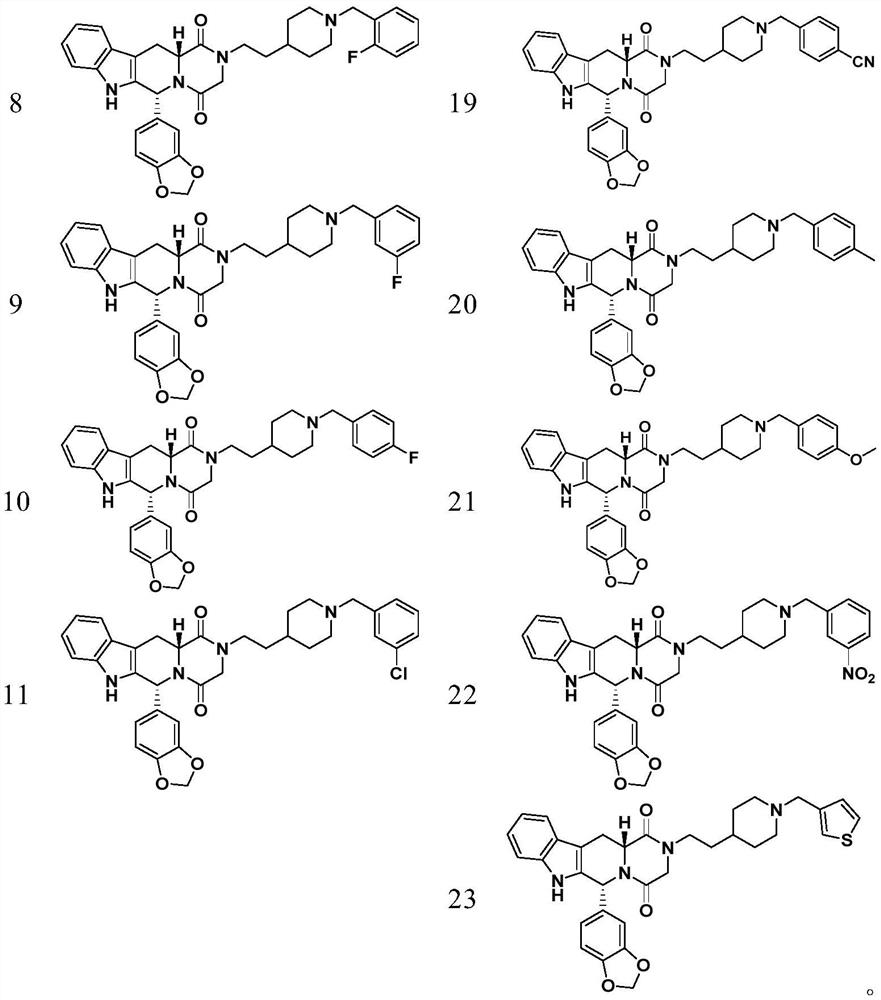
3,4-methylenedioxyphenyl substituted tetrahydro-β-carboline piperazine diketone derivatives and uses thereof
A phenyl, unsubstituted technology, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problem of terminating the disease process and single target
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The preparation method of the compound of the present invention is not limited to the specific preparation method described in the present invention, and can optionally be combined with various synthetic methods described in this description or known in the art to make it conveniently. Such a combination can be obtained by It is easy for those skilled in the art to which the present invention pertains.
[0056] Usually, in the preparation process, each reaction is usually carried out in an inert solvent at room temperature to reflux temperature (such as 0°C to 80°C, preferably 0°C to 50°C). The reaction time is usually 0.1-60 hours, preferably 0.5-48 hours.
[0057] The preparation method of the compound of the present invention may comprise the following steps:
[0058]
[0059] (1) In an inert solvent, carry out Pictet-Spengler reaction with piperonal and D-tryptophan methyl ester hydrochloride or L-tryptophan methyl ester hydrochloride, thereby forming the compou...
Embodiment 1
[0099] Example 1 Preparation of 3,4-methylenedioxybenzaldehyde
[0100]
[0101] Dissolve 1.521g (10mmol) of piperonyl alcohol in 20mL of dichloromethane, add 8.694g (100mmol) of active manganese dioxide, stir and react at room temperature for 16h, after the reaction is complete, filter with suction, and evaporate the solvent under reduced pressure to obtain a white solid 1.389 g, yield 93%.
[0102] 1 H NMR (400MHz, CDCl 3 )δ9.82(s,1H),7.42(dd,J=7.9,1.6Hz,1H),7.34(d,J=1.5Hz,1H),6.94(d,J=7.9Hz,1H),6.08( s,2H).
Embodiment 2
[0103] Example 2 (1R, 3R)-1-(3,4-methylenedioxyphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester (intermediate II-1) Preparation
[0104]
[0105] Add 900mg (6mmol) of piperonal, 1.528g (6mmol) of D-tryptophan methyl ester hydrochloride, and 20mL of isopropanol into a round-bottomed flask, heat and reflux for 24 hours, spin off the solvent after the reaction, and add an appropriate amount of Adjust the pH to 7-8 with saturated sodium bicarbonate solution, extract with ethyl acetate, wash with saturated brine, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure, separate and purify the residue by silica gel column chromatography, ethyl acetate / petroleum Ether = 1 / 3 was eluted to obtain 1.581 g of a yellow solid with a yield of 75%.
[0106] 1 H NMR (400MHz, Acetone-d 6 )δ9.54(s,1H),7.49(d,J=7.4Hz,1H),7.25(d,J=7.5Hz,1H),7.08–6.97(m,2H),6.95(d,J=7.9 Hz,1H),6.86(s,1H),6.82(d,J=7.9Hz,1H),5.98(s,2H),5.23(s,1H),3.93(dd,J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com