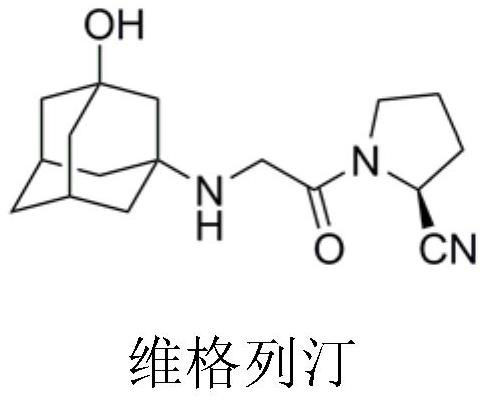
Preparation process of vildagliptin impurity
A vildagliptin impurity and process technology, applied in the field of drug synthesis, can solve the problems of expensive raw materials, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
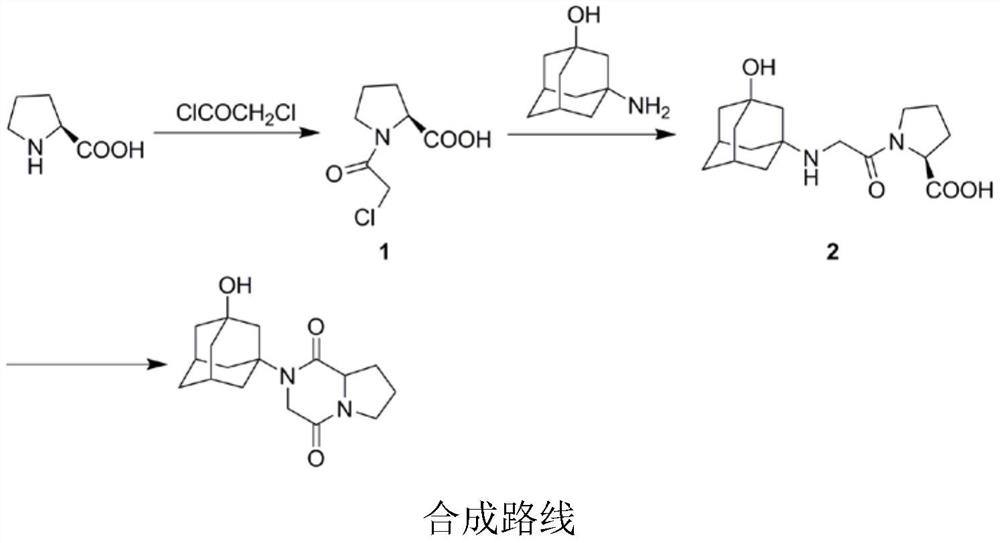
[0024] Preparation of Compound 1
[0025] 4g (0.034mol) L-proline is packed in the 100ml round-bottomed flask, and reaction bottle is placed under ice bath condition, N 2 Slowly add 11.05ml (0.138mol) of chloroacetyl chloride dropwise under protection into the reaction flask, and react for 10 minutes after the drop is completed. The temperature was raised to 70° C. and stirred under reflux for 1.5 h. After the reaction was completed, cool to room temperature, add 10ml of water to the reaction flask to dilute and stir for 20min. Then, 30 ml of saturated brine and 50 ml of ethyl acetate were added for extraction, and the organic layer was collected. The aqueous layer was repeatedly extracted with 60 ml of ethyl acetate. The organic layers were combined and dried by adding anhydrous sodium sulfate. After filtration, the filtrate was concentrated to obtain a pale yellow oil, which was crystallized by adding isopropyl ether dropwise under ice-bath conditions, and dried by sucti...
Embodiment 2
[0027] Preparation of Compound 2
[0028] Add 1.4g (0.008mol) of 3-amino-1-adamantanol, 1.7ml of triethylamine, and 0.15g of KI into 40ml of tetrahydrofuran, stir to dissolve, and control the temperature at 40°C. A solution of 2.3 g (0.012 mol) of compound 1 dissolved in 20 ml of tetrahydrofuran was slowly added dropwise through the dropping funnel, and added dropwise to the reaction flask at a constant speed within 30 min. After dripping, it was heated to reflux for 4 hours. After the reaction was completed, it was cooled to room temperature and filtered. The filtrate was distilled off under reduced pressure to remove the organic solvent. The residue was recrystallized with ethyl acetate, filtered and dried to obtain 1.88 g of compound 2 as a white solid, yield: 73.8%. m.p.200.8-203.4°C; ESI-MS (m / z): 323 [M+H]+.
Embodiment 3
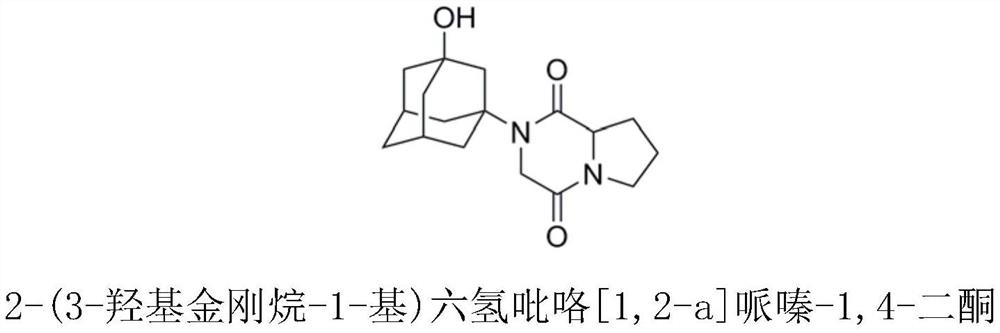
[0030] Preparation of vildagliptin impurity 2-(3-hydroxyadamantan-1-yl)hexahydropyrrole[1,2-a]piperazine-1,4-dione
[0031] Add 3.6g (0.01mol) of compound 2 and 40ml of DMF into a 100ml reaction flask and stir to dissolve, then add 2.02g of triethylamine dropwise under ice cooling. After dropping, the reaction bottle was transferred to -10°C, N 2 Under protection, slowly drop 2.73g (0.02mol) isobutyl chloroformate into the reaction flask. After dropping, keep at -10°C for 30 minutes, then rise to room temperature for 1 hour. After the reaction was completed, add 50ml of water to quench and continue to stir for 20min, extract with 50ml of ethyl acetate solution, collect the organic layer, then repeatedly extract the water layer with 60ml of ethyl acetate, combine the organic layers, and add anhydrous sodium sulfate to dry. Suction filtration, rotary evaporation to remove the organic solvent to obtain an oil, crystallization with acetone, filtration, and drying to obtain the i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com