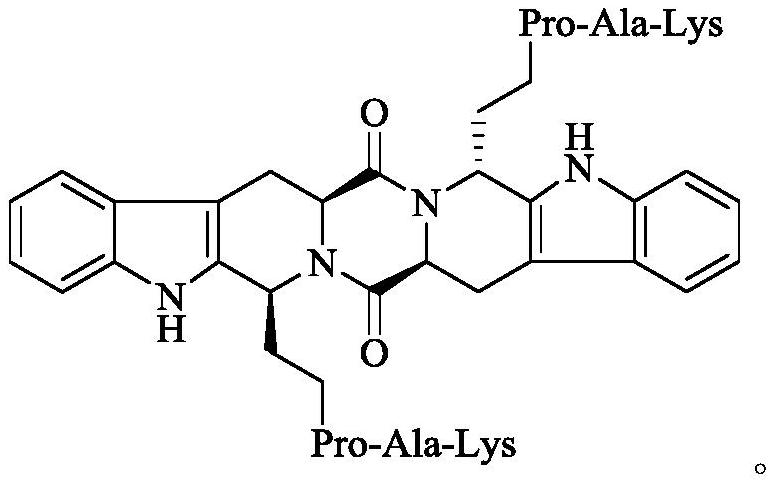
Ethyl PAK modified biscarboline piperazine diketone and preparation, activity and application thereof
A technology of diketone and ethyl, applied in the direction of medical preparations containing active ingredients, chemical instruments and methods, tripeptide components, etc., can solve problems such as no curative effect, chest cavity and abdominal cavity bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
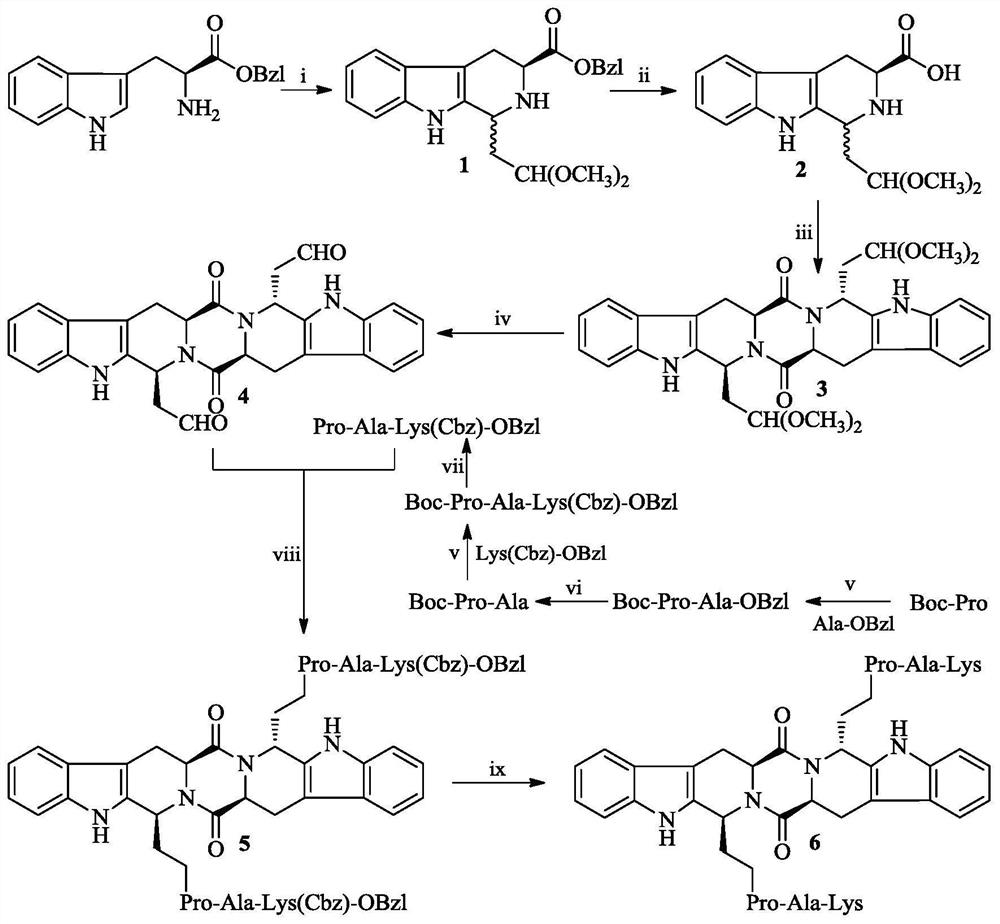
[0020] Example 1 Preparation of (3S)-1-(2,2-dimethoxyeth-1-yl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (1)
[0021] Add 5 mL of 1,1,3,3-tetramethoxypropane and 3.5 mL of trifluoroacetic acid to 150 mL of dichloromethane under stirring at 0°C, react for 40 minutes, then add 5 g (17.00 mmol) of Trp-OBzl, The reaction was carried out at 40°C. After reacting for 4 hours, the pH value of the reaction solution was adjusted to 7 with concentrated ammonia water at 0°C with stirring, concentrated under reduced pressure, and the residue was dissolved in 100 mL of dichloromethane, washed three times with saturated aqueous sodium bicarbonate solution, saturated chlorinated The sodium solution was washed 3 times, the dichloromethane phase was dried with anhydrous sodium sulfate for 12 hours, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain a yellow oil, which was purified by silica gel column chromatography to obtain 5.50 g (82%)...
Embodiment 2
[0022] Example 2 Preparation of (3S)-1-(2,2-dimethoxyeth-1-yl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid (2)
[0023] 5.50g (13.96mmol) (3S)-1-(2,2-dimethoxyeth-1-yl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl The ester (1) was dissolved in 50 mL of acetone, and NaOH aqueous solution (2M) was added thereto under stirring at 0° C. to adjust the pH value of the solution to 12, and reacted for 3 hours to keep the pH value of the reaction solution at 12. Thereafter, the pH was adjusted to neutral with saturated potassium hydrogensulfate aqueous solution, and concentrated under reduced pressure. 50 mL of acetone was added to the residue, the insoluble solid was removed by filtration, and the filtrate was concentrated to dryness under reduced pressure to obtain 3.63 g (86%) of the title compound as a yellow sticky substance. ESI-MS(m / e):303[M-H] - .
Embodiment 3
[0024] Example 3 Preparation of (2'S,5'S)-tetrahydropyrazine[1',2':1,6]bis[1S,1R-(1-dimethoxyeth-1-yl)-2,3,4 ,9-Tetrahydro-1H-pyridino[3,4-b]indole]-1',4'-dione (3)
[0025] 3.63g (11.94mmol) (3S)-1-(2,2-dimethoxyeth-1-yl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid (2 ) was dissolved in 20 mL of anhydrous N,N-dimethylformamide, and then 5.44 g (14.32 mmol) of 2-(7-azobenzotriazole)-N,N,N',N'-tetra Methylurea hexafluorophosphate, the solution was adjusted to pH 8 with N-methylmorpholine under stirring at 0°C, and after stirring at room temperature for 16 hours, 150 mL of distilled water was added to the reaction solution, followed by extraction with dichloromethane ( 50mL×3), combined the dichloromethane phase, and then washed with saturated aqueous sodium chloride solution (50mL×3), the dichloromethane phase was dried with anhydrous sodium sulfate for 12 hours, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain a yellow The oil was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com