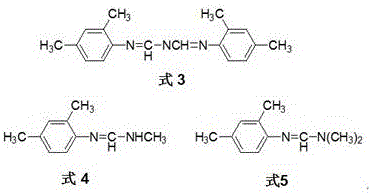
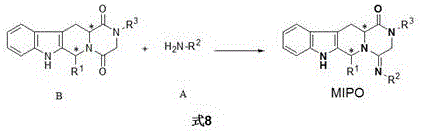
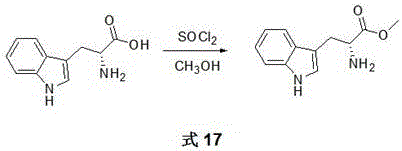
Synthesis and purpose of amidine compound containing two chiral centers
A compound and chiral technology, applied in the field of synthesis of amidine compounds, can solve the problem of great harm of peppers and achieve good economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Synthesis (6 R ,12a R )-2,3,6,7,12,12a-hexahydro-4-methylimino-2-methyl-6-(3,5-difluorophenyl)-pyrazin-1-one-[ 6,1:2',1']pyridine[3,4-b]indole (English name: (6 R ,12a R )-2,3,6,7,12,12a-Hexahydro-4-methylimino -2-methyl-6-(3,5-difulorophenyl)-piperazin-1-one-[6,1:2',1' ]pyrido[3,4-b]indole; MIPO-6-1 for short)
[0041] 1) Preparation of catalyst trimethylsilyl polyphosphate:
[0042] Under nitrogen protection, add 50ml of dried solvent dichloromethane, phosphorus pentoxide (14.2g, 50mmol), and hexamethyldisiloxane (25.6g, 160mmol) into a dry three-necked reaction flask, and heat to reflux. After reacting for 1 hour, the reflux reaction device was changed to a distillation reaction device, and the temperature was gradually raised to 160°C. During the heating process, the low boiling point solvent and unreacted hexamethyldisilyl ether were distilled out and kept at 160°C for 1 hour. The obtained syrupy liquid was 27.8 g (liquid is trimethylsilyl polyphosphate), i...
Embodiment 2
[0046] Example 2: Synthesis (6 R ,12a R )-2,3,6,7,12,12a-hexahydro-2-methyl-6-(3,5-difluorophenyl)-pyrazine-[6,1:2',1']pyridine [3,4-b]Indole-1,4-dione (English name: (6 R ,12a R )-2,3,6,7,12,12a-Hexahydro-2-methyl-6-(3,5-difuloro phenyl)-pyrazino-[6,1:2,1']pyrido[3,4-b ]indole-1,4-dione; referred to as B-6-1).
[0047]
[0048] The compound C-6-1((1R, 3R)methyl 2-(2-chloroacetyl)-1-(3,5-difluorophenyl) -2,3,4,9-tetrahy dro-1H- pyrido[3,4 -b]indole-3-carboxylate) (16.7g, 40mmol) was dissolved in 160 mL DMF. At room temperature, slowly add 30% methylamine aqueous solution (20.7g, 200mmol) to the solution, and the addition is complete Afterwards, it was stirred overnight at room temperature and monitored by thin layer chromatography. When the reaction of the raw material C-6 was complete, the reaction was stopped. Add 500 grams of ice-water mixture to another reaction flask, add the above reaction liquid dropwise to the ice-water mixture under vigorous stirring, let stand, filter...
Embodiment 3
[0049] Example 3: Synthesis of (1R, 3R)-2-(2-chloroacetyl)-1-(3,5-difluorophenyl)-2,3,4,9-tetrahydro-1H-pyridine [3 ,4-b)Indole-3-carboxylic acid methyl ester (English name: (1R, 3R)-methyl 2-(2-chloroacetyl)-1- (3,5-difluorophenyl)-2,3,4,9 -tetrahydro-1H-pyrido [3,4-b] indole -3-carboxylate; referred to as C-6-1).
[0050]
[0051] In a three-necked flask equipped with mechanical stirring, add E-6-1((1R, 3R)-methyl 1-(3,5-difluorophenyl)-2,3,4,9-tetrahydro- 1H-pyrido[3 ,4-b]indole-3-carboxylate) hydrochloride (37.9 g, 100 mmol), triethylamine (30.4 g, 300 mmol), 500 mL of dichloromethane, stir at room temperature for half an hour, the solution becomes clear and transparent. Using an ice-salt bath, the temperature of the reaction system was lowered to 0°C, chloroacetyl chloride (16.9 g, 150 mmol) was added dropwise, and the addition was kept at 0-5°C. After the addition is completed, the reaction is stirred at 0~5°C and monitored by thin layer chromatography. When the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com