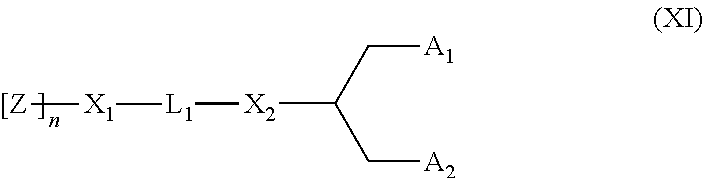
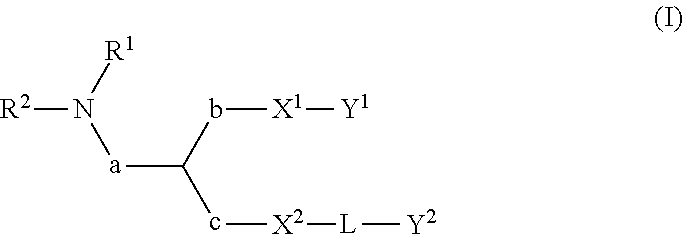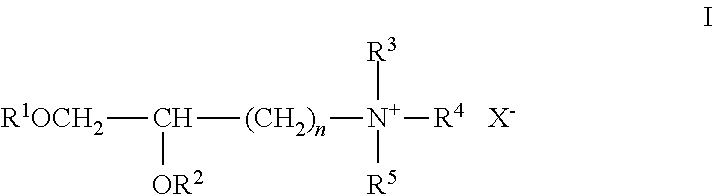Lipids, lipid compositions, and methods of using them
a technology of lipid compositions and lipid compounds, applied in the direction of drug compositions, peptide/protein ingredients, antibody medical ingredients, etc., can solve the problems of high trafficking of biologically active agents into living cells, affecting the delivery of biologically active agents to subjects, and increasing the risk of toxic effects and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0640]Linoleyl alcohol (48.7 g, 183 mmol) is added to a round bottom flask and dissolve in THF (400 mL). The resulting solution is cooled using an ice bath and sodium hydride (13.16 g, 329 mmol) is added. The resulting slurry is stirred for 1 h at rt. Epibromohydrin is added in one portion and the reaction is continued at rt. After 4 h stirring, additional sodium hydride (13.16 g, 329 mmol) is added. After an additional h stirring at rt, another aliquot of epibromohydrin (32.5 g, 238 mmol) is added. The reaction is then heated to 50° C. overnight. The reaction is then cooled to rt and quenched with water. EtOAc is added and the resulting organic layer collected, washed with brine and dried over sodium sulfate. The volatiles are removed by rotary evaporation and the resulting residue purified by chromatography on silica in EtOAc / heptanes to yield the desired epoxide.
Cholesterol Tosylate
example 2
[0641]Cholesterol (50 g, 129 mmol) is dissolved in DCM (55 mL) and pyridine (150 mL). The resulting solution is cooled to 0° C. and tosyl chloride is added in one portion as a solid. The reaction is allowed to slowly warm to rt overnight. The reaction is concentrated by rotary evaporation and MeOH (500 mL) is added to produce a white solid. Stirring is continued for 30 min and the precipitate collected by filtration, washed with MeOH and dried under vacuum to yield the desired tosylate.
Cholesterol Diglycol
example 3
[0642]Cholesterol tosylate (73 g, 128 mmol) is dissolved in 1,4-dioxane (750 mL). Diethylene glycol (294 mL, 3077 mmol) is added and the reaction is heated to a gentle reflux overnight. The resulting solution is cooled to rt and concentrated by rotary evaporation. The resulting gel is taken up in DCM and stirred with water. The resulting organic layer is collected and the aqueous layer extracted once with DCM. The organic layers are combined, dried over sodium sulfate, and concentrated by rotary evaporation. The crude product is purified on silica in EtOAc / heptane to yield the desired cholesterol diglycol.
Cholesterol Diglycol Tosylate
PUM
| Property | Measurement | Unit |
|---|---|---|
| pKa | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com