Application of dexrazoxane in preparation of drug for treating neurodegenerative diseases
A technology of neurodegenerative and dextropropanimine, which is applied in nervous system diseases, drug combinations, pharmaceutical formulations, etc., can solve the problem of no reports of dextropropimine in the treatment of neurodegenerative diseases, etc., and achieve the reduction of α-synuclear Protein deposition, huge market value and social benefits, the effect of improving behavioral disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
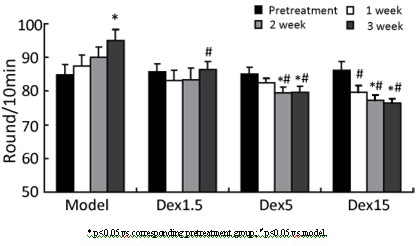
[0035] Example 1 Dextropropanimine improves the behavioral symptoms of 6-hydroxydopamine-induced Parkinson's disease model rats
[0036] 1.1 Experimental materials
[0037] 7-8 weeks old, 180-220g healthy male SD rats. Feeding conditions included standard feed, tap water, room temperature maintained at (24 ± 2) °C, humidity 50-60%, and daily light and dark times of 12 hours each. Before the experiment, the animals were placed in the experimental environment for 3 days to adapt.
[0038]Dextropropylimine (DEX) was donated by Jiangsu Aosaikang Pharmaceutical Co., Ltd. 6-Hydroxydopamine (6-OHDA) and apomorphine (apomorphine) were purchased from Sigma (St. Louis, MO, USA), and both were prepared in normal saline. Aliquots of 6-OHDA were stored at -20 °C, and apomorphine was freshly prepared within 30 min before administration. Brain stereotaxic instrument was purchased from Stoelting Company of the United States.
[0039] 1.2 Establishment of animal models
[0040] Anesthe...
Embodiment 2
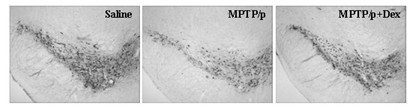
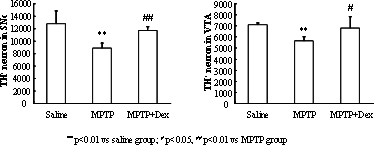
[0045] Example 2 Neuroprotective Effect of Dextropropanimine on Chronic MPTP / p Nerve Injury Model Mice
[0046] 2.1 Experimental materials
[0047] 3-4 months old, healthy male C57BL / 6J mice. Feeding conditions included standard feed, tap water, room temperature maintained at (24 ± 2) °C, humidity 50-60%, and daily light and dark times of 12 hours each. Before the experiment, the animals were placed in the experimental environment for 3 days to adapt.
[0048] Dextropropylimine (DEX) was donated by Jiangsu Aosaikang Pharmaceutical Co., Ltd. MPTP was purchased from Sigma (St. Louis, MO, USA), and probenecid was purchased from Jinan Times Pharmaceutical Technology Co., Ltd. DEX was prepared with normal saline, prepared into a stock solution and stored at -20 °C, and the prepared stock solution was diluted with normal saline to the required concentration before use. MPTP was prepared with normal saline within 30 min before administration and kept on ice. Probenecid was pre...
Embodiment 3
[0058] Example 3 Dextropropanimine to MPP + induce Protective effect of SH-SY5Y cell injury
[0059] 3.1 Experimental materials
[0060] SH-SY5Y cell line: dopaminergic neuron cell line, purchased from Union Cell Institute, Chinese Academy of Medical Sciences. Dextropropylimine (DEX) was donated by Jiangsu Aosaikang Pharmaceutical Co., Ltd. MPP + purchased from Sigma (St. Louis, MO, USA); H 2 o 2 and methazolium blue (MTT) were purchased from Guangzhou Chemical Reagent Factory; microplate reader was purchased from Thermo Company.
[0061] 3.2 Experimental method
[0062] Cells were seeded on 96-well plates and cultured for 24 hours, then the culture medium was replaced, and the cells were randomly divided into groups. Pretreatment with different concentrations of DEX for 30 min, followed by H 2 o 2 (300 μM, 2h) or MPP + (500 μM, 48h), 20 μl of 5 mg / ml MTT solution was added to each well, and incubation was continued for 4 h before the culture was terminated. Car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com