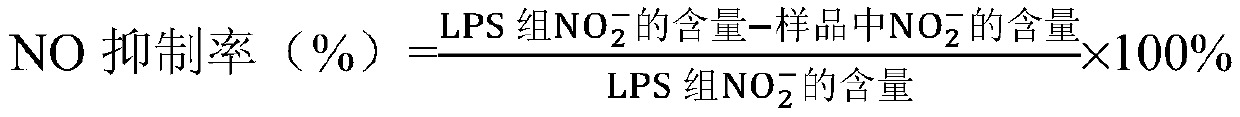A kind of pharmaceutical composition, its preparation method and application
A technology of inhibitors and sediments, which is applied in the field of pharmacy, can solve the problems such as the prevention and treatment of neurodegenerative diseases without polysaccharides in leaves, and achieves the effects of remarkable medicinal effect, simple preparation process and inhibition of damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 Preparation of the pharmaceutical composition of the present invention
[0038] The extraction method of the southern yew extract comprises the following steps:
[0039]Take the southern yew leaves, add 10 times the amount of water to decoct three times, 2 hours each time, combine the decoction liquid, and cool to room temperature; add a precipitant to the decoction liquid until the volume ratio of the precipitant reaches 60%, and filter after standing Obtain a precipitate; mix the precipitate with water, centrifuge, deproteinize the supernatant with 15% trichloroacetic acid aqueous solution at 4°C, and centrifuge to obtain a supernatant; add a precipitant to the obtained supernatant, and filter to obtain a precipitate , washed with absolute ethanol and then dried in vacuum at 50° C. to obtain the southern yew leaf extract.
[0040] The extracted southern yew leaf extract is mixed with polyethylene glycol to make capsule medicine.
experiment example 2
[0041] Experimental example 2 Verification of inhibition of microglia activation
[0042] BV2 cells are mouse microglial cell lines;
[0043] Griess reagent: prepare 0.1% naphthalene ethylenediamine with distilled water, prepare 1% p-aminobenzenesulfonic acid with 5% phosphoric acid, mix the two in equal volume 1:1 before use;
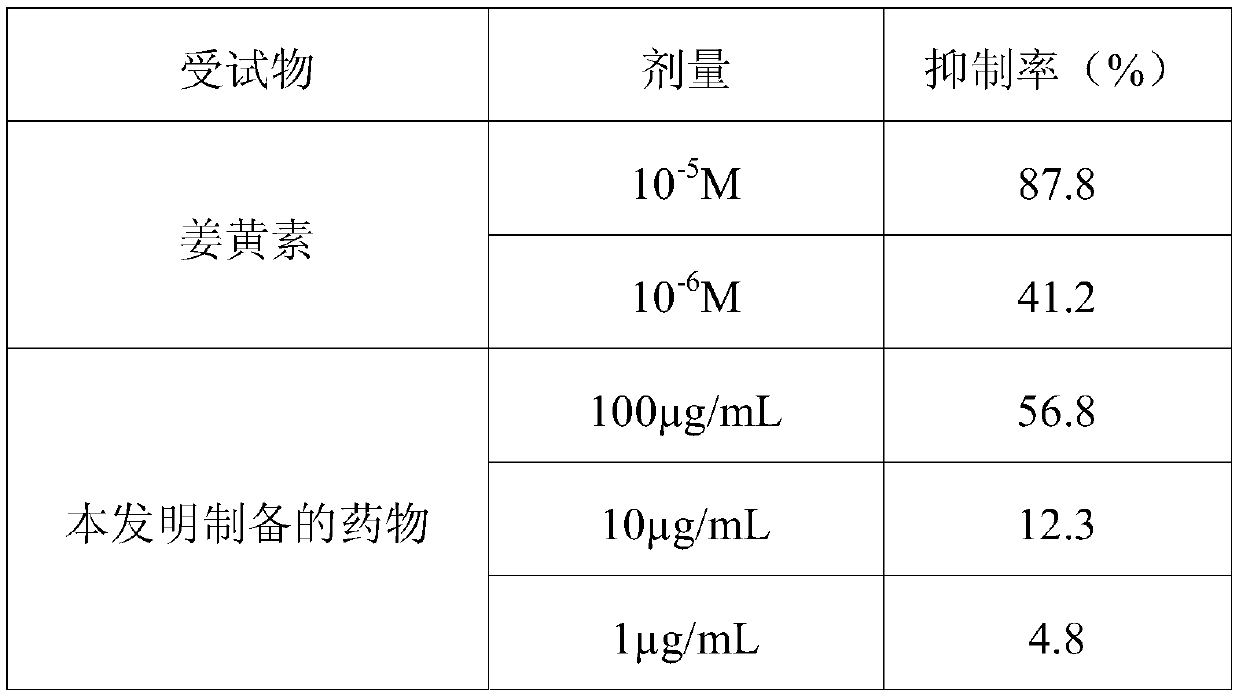
[0044] Curcumin, formulated in DMSO;
[0045] Lipopolysaccharide LPS, prepared with sterile PBS, the final concentration is 300ng / mL;
[0046] Experimental method: (1) BV2 cells were cultured in DMEM-F12 medium containing 10% newborn bovine serum at 37°C, 5% CO 2 , 95% air, 100% relative humidity growth;
[0047] (2) BV2 cells in the logarithmic growth phase, after digesting and counting, were divided into 2×10 4 Inoculate each well into a 96-well plate, add the test substance 24 hours later, add LPS 1 hour later, the final concentration is 300ng / mL, continue to cultivate for 24 hours, and collect the medium supernatant;
[0048] (3) Take 100 μL o...
experiment example 3
[0054] Experimental example 3 Inhibition of neuroinflammation and neuron damage caused by LPS
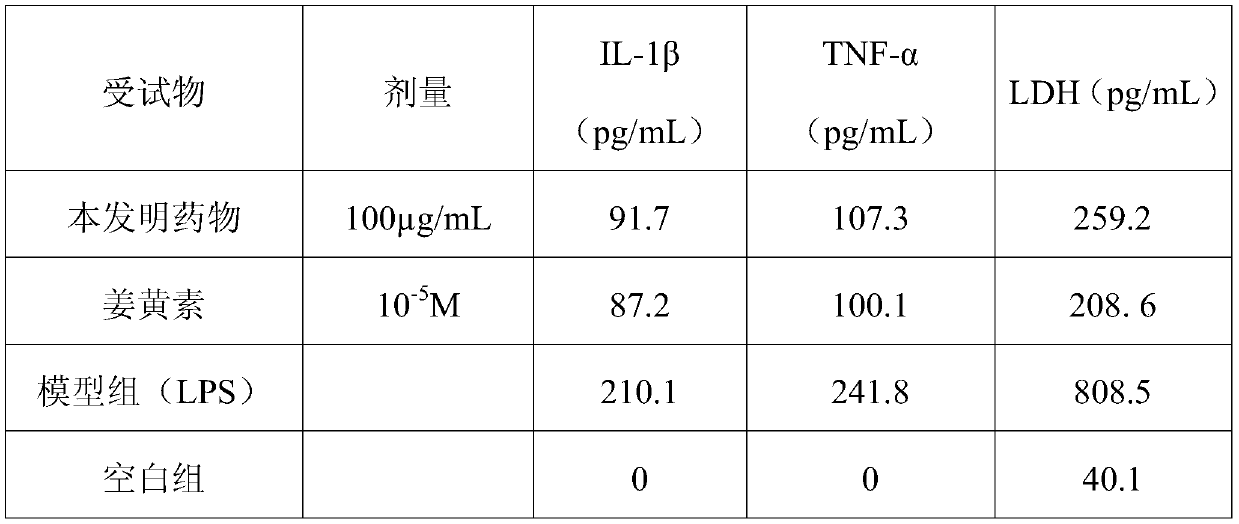
[0055] Lipopolysaccharide, prepared with sterile PBS, the final concentration is 100ng / mL;
[0056] Curcumin formulated with DMSO;
[0057] experimental method:
[0058] (1) Establishment of the mixed culture system of primary hippocampal neurons / glial cells: The hippocampus was isolated under a dissecting microscope from SD rat embryos at 18 days pregnant, blown and beaten with a pipette until no tissue blocks could be seen, and filtered. Inoculated in 24-well plates;
[0059] (2) Cell administration treatment: After the above-mentioned primary cells were cultured for 7 days, they were co-incubated with the test substance respectively. After 3 hours, the stimulating agent LPS 100 ng / mL was added. After 5 hours, the culture medium was taken to measure IL-1β and TNF- α;
[0060] (3) After the cells were treated with drugs for 7 days, the medium was collected to detect LDH with LD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com