Dexrazoxane-containing composition and preparation method thereof, and dexrazoxane freeze-drying preparation and redissolving solvent thereof
A technology of dextropropylimine and freeze-dried preparation, applied in the field of medicine, can solve the problems of purification process and purification process burden, high production cost, influence on production process and the like, achieves good clinical reconstitution effect, fast dissolution speed, and finished product related problems. Substance small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
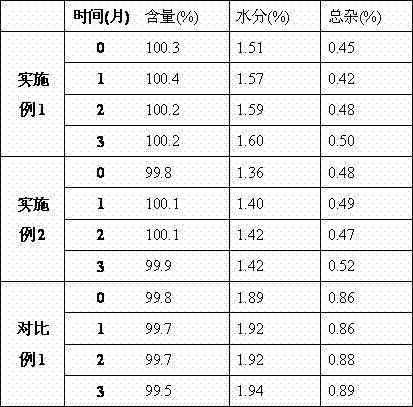
Examples
Embodiment 1
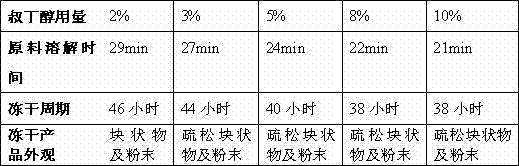
[0052] Add 8L of water for injection and 0.5L of tert-butanol to the mixing tank, cool down the water for injection in the mixing tank to 4°C~8°C, add the calculated amount of hydrochloric acid to make a 0.1mol / L hydrochloric acid solution. Slowly put 250g of dextropropylimine into the batching tank, adjust the pH value of the liquid to 1.8-2.0 with 1mol / L hydrochloric acid solution under stirring, after the raw materials are dissolved, add wetted activated carbon, add Water for injection to 10L, stirred for 20 minutes, decarbonized and sterilized by filtration; the filtrate was evenly distributed in 1000 vials and freeze-dried.
[0053] Freeze-drying curve: lower the temperature of the product to -45~-35°C and keep it warm for 2 hours. When the cold trap is cooled below -40°C, turn on the vacuum pump. When the vacuum degree drops below 20Pa, start to heat up; set the heat conduction oil to heat up to 25°C within 5 minutes, and keep it warm for about 30 hours. Shut down, pre...
Embodiment 2
[0056] Add 7L of water for injection and 0.8L of tert-butanol into the mixing tank, cool down the water for injection in the mixing tank to about 4°C, add the calculated amount of hydrochloric acid to form a 0.15mol / L hydrochloric acid solution. Slowly put 250g of dextropropylimine into the batching tank, adjust the pH value of the liquid to 1.5-1.8 with 1mol / L hydrochloric acid solution under stirring, after the raw materials are dissolved, add wetted activated carbon, and add water for injection below 10°C to 10L, stirred for 20 minutes, decarbonized and sterilized by filtration; the filtrate was evenly distributed in 1000 vials and freeze-dried.
[0057] Freeze-drying curve: lower the temperature of the product to -40~-30°C and keep it warm for 3 hours. When the cold trap is cooled below -40°C, turn on the vacuum pump. When the vacuum degree drops below 25Pa, start to heat up; set the heat transfer oil to 20°C within 10 minutes, and keep it warm for about 35 hours. Shut d...
Embodiment 3
[0060] Add 8L of water for injection and 0.8L of tert-butanol to the mixing tank, cool down the water for injection in the mixing tank to about 4°C, add the calculated amount of hydrochloric acid to form a 0.05mol / L hydrochloric acid solution. Slowly put 250g of dextropropylimine into the batching tank, adjust the pH value of the liquid to 1.8-2.0 with 1mol / L hydrochloric acid solution under stirring, after the raw materials are dissolved, add wetted activated carbon, and add water for injection at about 6°C to 10L, stirred for 20 minutes, decarbonized and sterilized by filtration; the filtrate was evenly distributed in 500 vials and freeze-dried.
[0061] Freeze-drying curve: lower the temperature of the product to -45~-35°C and keep it warm for 3 hours. When the cold trap is cooled below -40°C, turn on the vacuum pump. When the vacuum degree drops below 30Pa, start to heat up; set the heat conduction oil to heat up to 30°C within 10 minutes, and keep it warm for about 30 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com