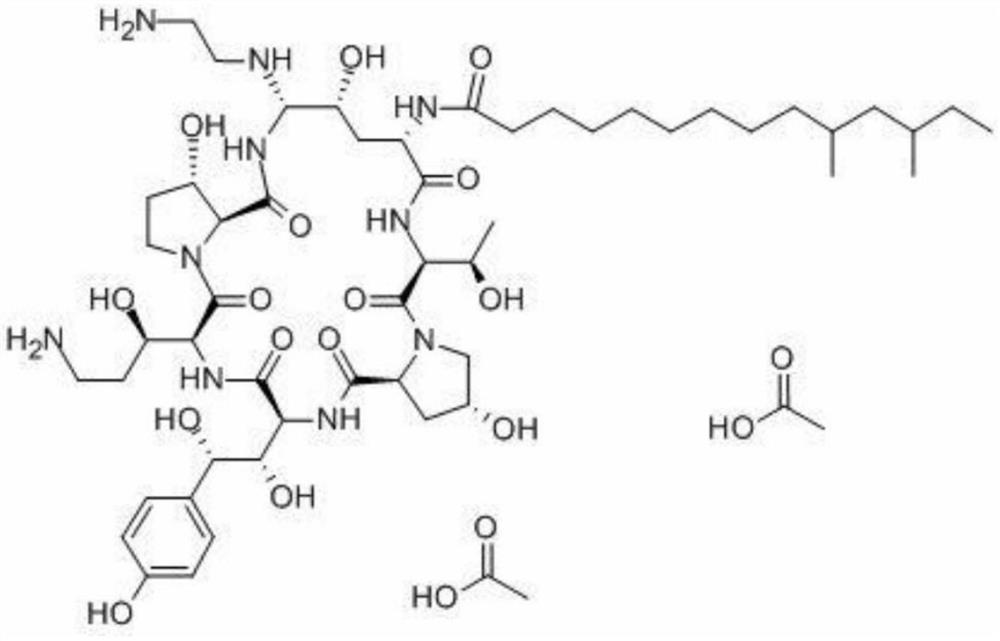
A kind of lyophilized composition containing caspofungin acetate and its preparation method
A technology of caspofungin acetate and composition, which is applied in the field of freeze-dried composition containing caspofungin acetate and its preparation, can solve the problems of unfavorable actual production, long total cycle of freeze-drying, and low drying efficiency, and achieve Energy saving, short freeze-drying cycle, and avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
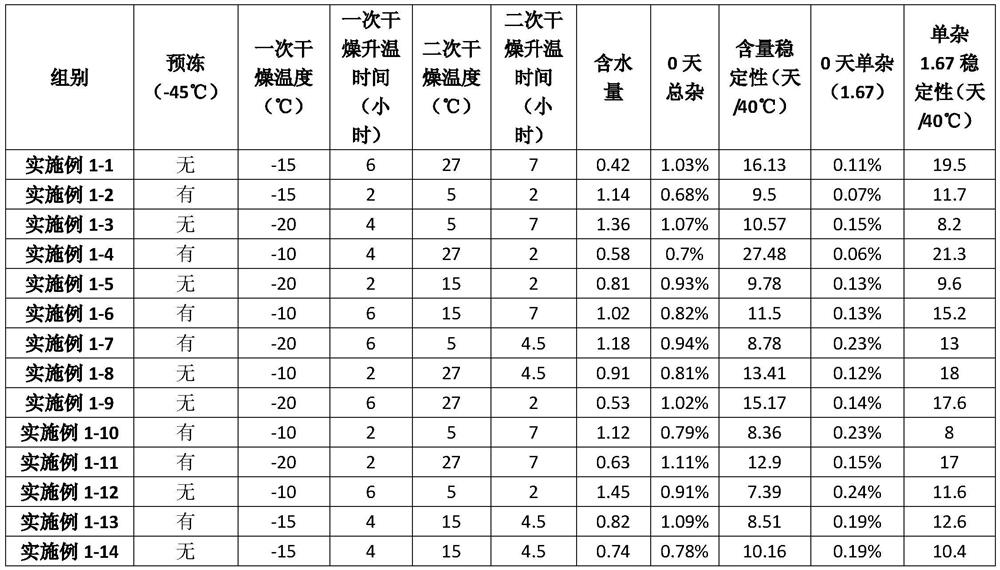
Examples
Embodiment 1
[0031] prescription:
[0032]
[0033] Preparation:
[0034] (1) Preparation of solution for lyophilization:
[0035] ① Solution preparation: Add water for injection to the preparation container, cool to 8°C, add the prescribed amount of mannitol and sucrose, stir to dissolve, add glacial acetic acid and caspofungin acetate, stir to dissolve, and use 0.3mol / l sodium hydroxide solution Adjust the pH value to 5.8, continue to add water for injection to 1.3 g, and stir until the solution is clear by visual inspection.
[0036] ② Bacterial endotoxin removal: Add 0.3% medicinal charcoal to the above solution and stir for 25 minutes.
[0037] ③Decarbonization: Decarbonization by coarse filtration with titanium rods.
[0038] ④ Sterilization and filtration of medicinal liquid: the medicinal liquid is filtered through three 0.22μm filter membranes connected in series.
[0039] ⑤Inspection: Inspect the intermediate content, pH value and visible foreign matters.
[0040] ⑥Fillin...
Embodiment 2
[0047] prescription:
[0048]
[0049] Preparation method:
[0050] (1) Preparation of solution for lyophilization
[0051] ① Solution preparation: Add water for injection to the preparation container, cool to 2°C, add the prescribed amount of mannitol and sucrose, stir to dissolve, add glacial acetic acid and caspofungin acetate, stir to dissolve, and use 0.3mol / l sodium hydroxide solution Adjust the pH value to 5.0, continue to add water for injection to 1.3 g, and stir until the solution is clear by visual inspection.
[0052] ②-⑥ same as embodiment 1
[0053](2) Freeze-drying: the freeze-drying solution obtained in step (1) is placed in a freeze-drying machine, and the freeze-drying process is as follows:
[0054] ①The shelf temperature drops to -45°C;
[0055] ② The shelf temperature is maintained at -45°C for 3 hours;
[0056] ③ Turn on the vacuum, the vacuum degree drops to 0.1mbar, the shelf temperature rises to -30°C within 0.5 hours, then rises to -20°C withi...
Embodiment 3
[0063] prescription:
[0064]
[0065] Preparation method:
[0066] (1) Preparation of solution for lyophilization
[0067] ① Solution preparation: Add water for injection to the preparation container, cool to 6°C, add the prescribed amount of mannitol and sucrose, stir to dissolve, add glacial acetic acid and caspofungin acetate, stir to dissolve, and use 0.3mol / l sodium hydroxide solution Adjust the pH value to 6.2, continue to add water for injection to 1.3 g, and stir until the solution is clear by visual inspection.
[0068] ②-⑥ same as embodiment 1
[0069] (2) Freeze-drying: the freeze-drying solution obtained in step (1) is placed in a freeze-drying machine, and the freeze-drying process is as follows:
[0070] ①The shelf temperature drops to -45°C;
[0071] ② The shelf temperature is maintained at -45°C for 3 hours;
[0072] ③ Turn on the vacuum, the vacuum degree drops to 0.1mbar, the shelf temperature rises to -30°C within 0.5 hours, then rises to -20°C with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com