A kind of famotidine freeze-dried powder injection
A technology for freeze-dried powder injection and famotidine, which is applied in the field of famotidine freeze-dried powder for injection, can solve the problems of non-compliance with pharmacopoeia regulations, high medication risk, and high cost, and achieves avoiding adsorption influence, good solid forming, and high cost. The effect of reducing water content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
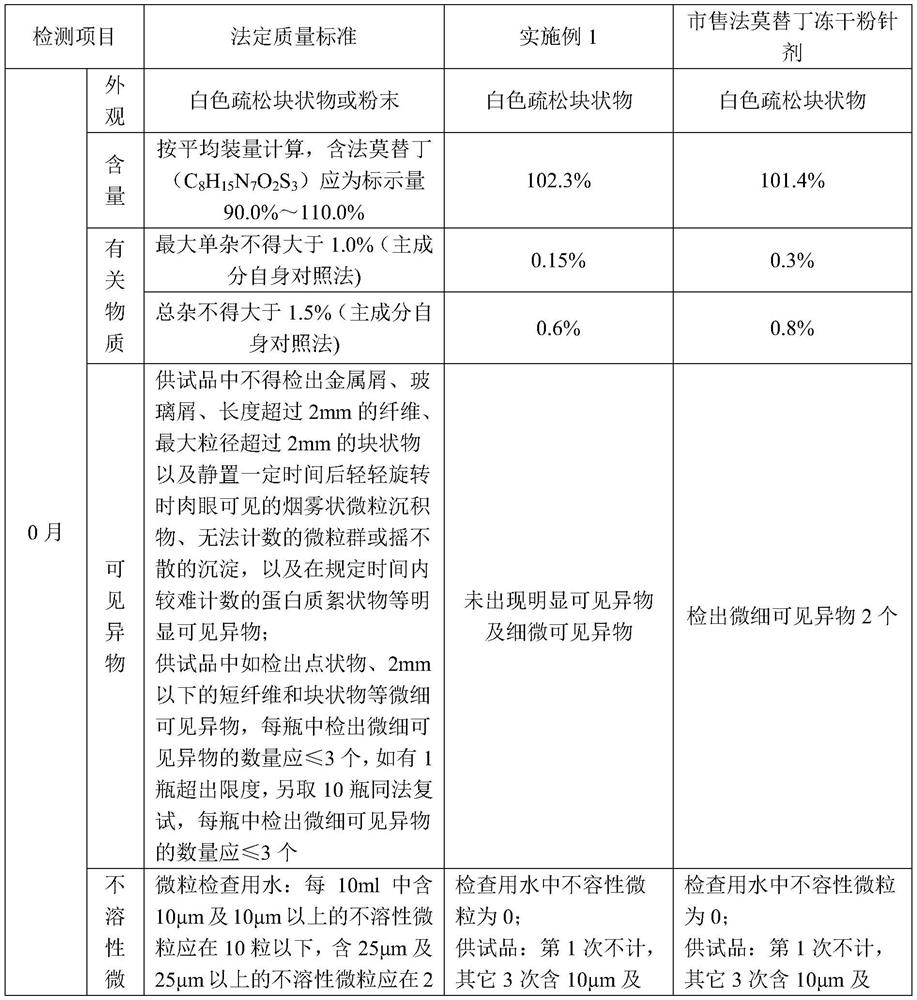
[0025] A famotidine freeze-dried powder for injection, the famotidine freeze-dried powder for injection is prepared by a method comprising the following steps:
[0026] S1. Add 0.44g of aspartic acid to 110.0g of water for injection with a temperature of 70-80°C, keep stirring for 15min to complete dissolution, add famotidine 1.1g and stir to complete dissolution for 15min, then add mannitol 1.1g After stirring for 5min to complete dissolution, add aqueous hydrochloric acid solution with a concentration of 1mol / L to adjust pH=5.0-5.6, then add water for injection to 110ml, and then filter and sterilize through 0.45μm and 0.22μm filters to obtain solution 1;
[0027] S2. Pack the solution 1 into a vial, then quickly freeze to -35°C, and keep the temperature for 120min; then perform sublimation once, and then perform secondary drying, and finally adjust the pressure to 4-5.5MPa for tamponade to obtain famotir Ding freeze-dried powder for injection, wherein, the procedure of one ...
Embodiment 2
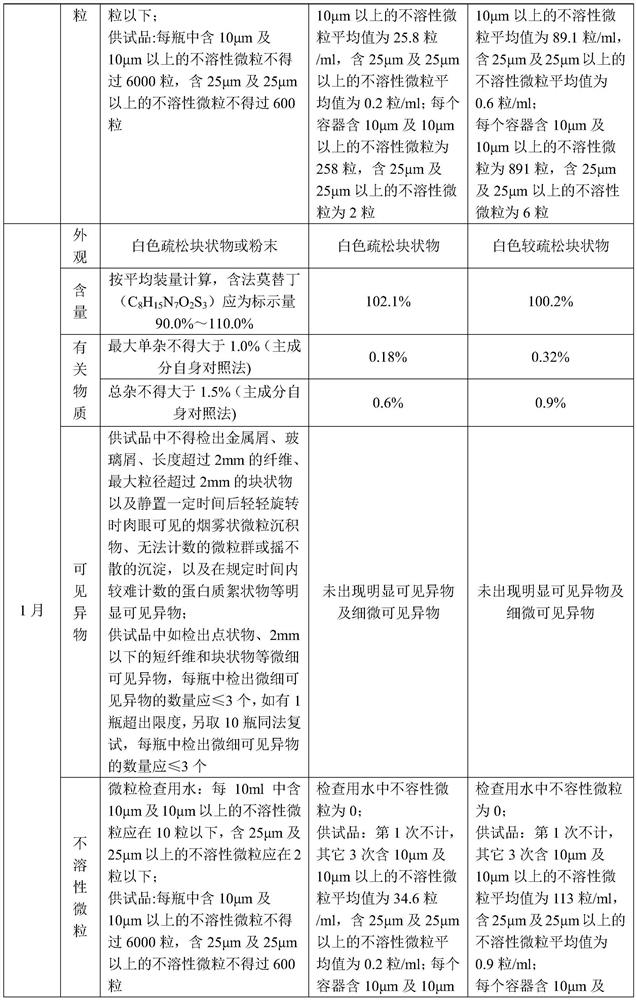
[0030] A famotidine freeze-dried powder for injection, the famotidine freeze-dried powder for injection is prepared by a method comprising the following steps:
[0031] S1. Add 0.44g of aspartic acid to 110.0g of water for injection with a temperature of 70-80°C, keep stirring for 15min until completely dissolved, add 1.1g of famotidine and stir until completely dissolved, then add 1.045g of mannitol After stirring for 5min to complete dissolution, add aqueous hydrochloric acid solution with a concentration of 1mol / L to adjust pH=5.0-5.6, then add water for injection to 110ml, and then filter and sterilize through 0.45μm and 0.22μm filters to obtain solution 1;
[0032] S2. Pack the solution 1 into a vial, then quickly freeze it to -37°C, and keep the temperature for 120min; then perform sublimation once, then perform secondary drying, and finally adjust the pressure to 4-5.5MPa for tamponade to obtain famotir Ding freeze-dried powder injection, wherein, the procedure of one s...
Embodiment 3
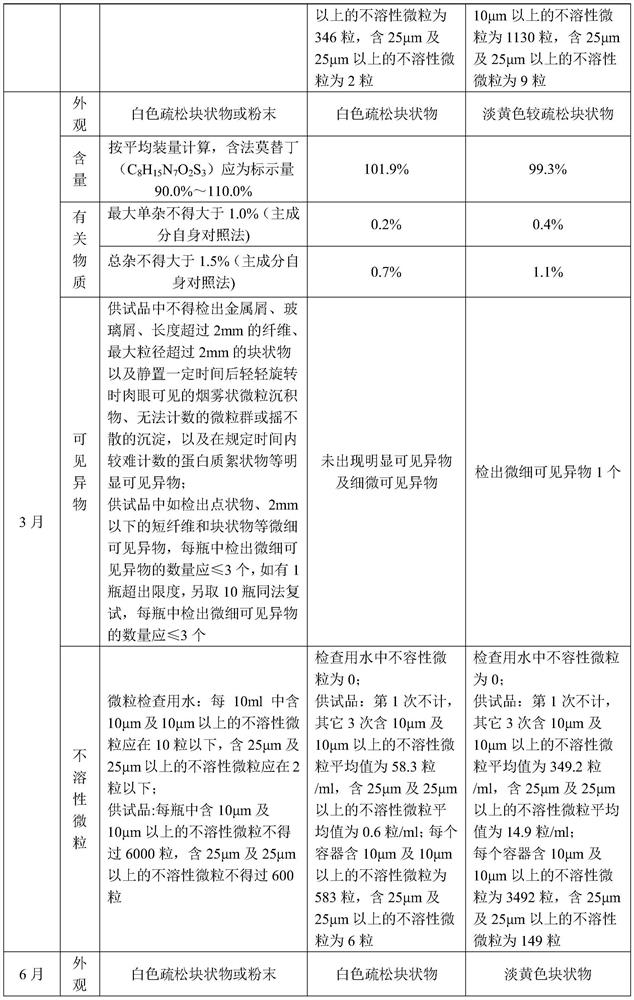
[0035] A famotidine freeze-dried powder for injection, the famotidine freeze-dried powder for injection is prepared by a method comprising the following steps:
[0036] S1. Add 0.44g of aspartic acid to 110.0g of water for injection with a temperature of 70-80°C, keep stirring for 15min until completely dissolved, add 1.1g of famotidine and stir until completely dissolved, then add 1.155g of mannitol After stirring for 5min to complete dissolution, add aqueous hydrochloric acid solution with a concentration of 1mol / L to adjust pH=5.0-5.6, then add water for injection to 110ml, and then filter and sterilize through 0.45μm and 0.22μm filters to obtain solution 1;
[0037] S2. Pack the solution 1 into a vial, then quickly freeze to -33°C, and keep the temperature for 120 minutes; then perform sublimation once, and then perform secondary drying, and finally adjust the pressure to 4-5.5MPa for tamponade to obtain famotir Ding freeze-dried powder for injection, wherein, the procedur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com