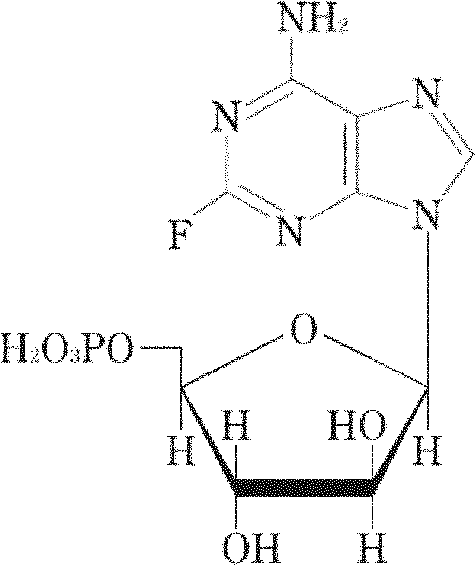
Fludarabine phosphate freeze-dried powder injection and preparation method thereof
A technology for fludarabine phosphate and freeze-dried powder injection, which is applied in the field of fludarabine phosphate freeze-dried powder injection and its preparation, can solve the problem of increasing the type and content of impurities in freeze-dried powder injection, and the thermal stability of fludarabine phosphate. It can solve problems such as sexual influence and poor thermal stability of fludarabine phosphate, and achieve the effect of excellent stability, good clarity and full appearance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Add 1530ml of water for injection into the liquid mixing tank, and the temperature of the liquid mixing tank is 10°C, then add 50g of fludarabine phosphate, after fully stirring, add an appropriate amount of 5mol / L sodium hydroxide solution and stir until the fludarabine phosphate is completely Dissolve; then add 40g of mannitol, stir to make the medicinal solution into a clear solution; adjust the pH value of the medicinal solution to 7.7 with 5mol / L sodium hydroxide solution, add 270ml of water for injection, and mix well.
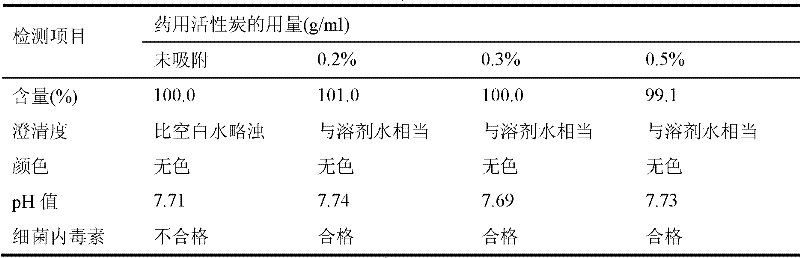
[0050] Add 5.4g of activated carbon to the clear solution, stir and absorb for 30 minutes, use a 0.45μm microporous filter membrane to filter and decarburize while decarburizing, 0.2μm filter membrane for primary sterilization and filtration, and 0.2μm filter membrane for secondary terminal Sterilize and filter, and put the obtained filtrate into the liquid medicine bottle for filling.
[0051] The filtrate is packed separately, each bottle contai...
Embodiment 2
[0057] Add 1360ml of water for injection into the liquid mixing tank, the temperature of the liquid mixing tank is 0°C, then add 50g of fludarabine phosphate, after fully stirring, add an appropriate amount of 5mol / L sodium hydroxide solution and stir until the fludarabine phosphate is completely Dissolve; then add 30g of mannitol, stir to make the medicinal solution into a clear solution; adjust the pH value of the medicinal solution to 7.2 with 5mol / L sodium hydroxide solution, add 240ml of water for injection, and mix well.
[0058] Add 4.8g of activated carbon to the clear solution, stir and absorb for 30 minutes, use a 0.45μm microporous filter membrane to filter and decarburize while decarburizing, 0.2μm filter membrane for one-time sterilization filtration and 0.2μm filter membrane for secondary terminal Sterilize and filter, and put the obtained filtrate into the liquid medicine bottle for filling.
[0059] The filtrate is packed separately, each bottle contains 25 mg ...
Embodiment 3
[0065] Add 1700ml of water for injection into the liquid mixing tank, the temperature of the liquid mixing tank is 15°C, then add 50g of fludarabine phosphate, after stirring thoroughly, add an appropriate amount of 5mol / L sodium hydroxide solution and stir until the fludarabine phosphate is completely Dissolve; then add 50g of mannitol, stir to make the medicinal solution into a clear solution; adjust the pH value of the medicinal solution to 8.2 with 5mol / L sodium hydroxide solution, add 300ml of water for injection, and mix well.
[0066]Add 6g of activated carbon to the clear solution, stir and absorb for 30 minutes, use a 0.45μm microporous filter membrane to filter and decarburize while decarburizing, 0.2μm filter membrane for primary sterilization and filtration, and 0.2μm filter membrane for secondary terminal removal Bacterial filtration, and the obtained filtrate is put into the liquid medicine bottle for filling.
[0067] The filtrate is packed separately, each bott...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com