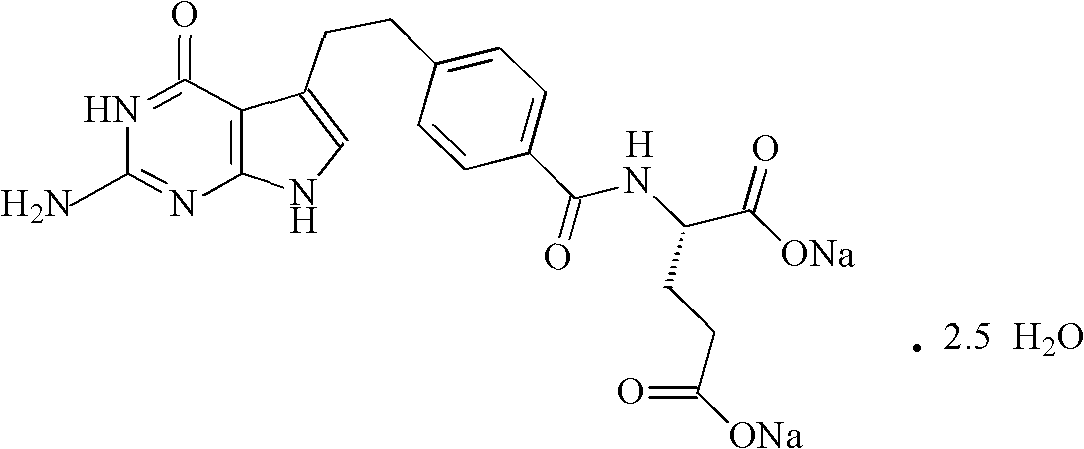
Pemetrexed disodium freeze-dried powder injection and preparation method thereof
A technology of pemetrexed disodium and freeze-dried powder injection, which is applied in the field of medicine, can solve the problems of rough freeze-drying process, increased risk, and no public improvement, and achieve the effect of avoiding adverse effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
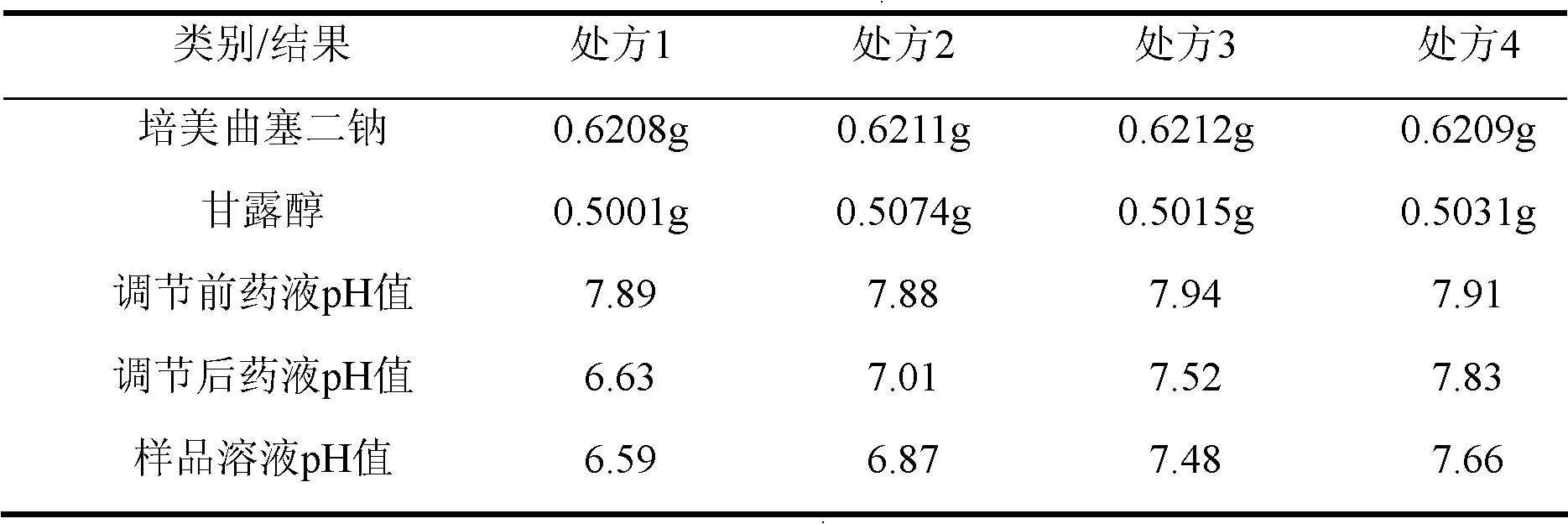
[0047] Add 2244ml of water for injection into the liquid preparation tank, the temperature of the liquid preparation tank is 5°C, then add 80g of pemetrexed disodium, and stir to dissolve. Then add 96g of mannitol, stir to make the liquid into a clear solution; use 0.1mol / L hydrochloric acid solution and / or 0.1mol / L sodium hydroxide solution to adjust the pH value of the liquid to 7.3, add 396ml of water for injection, and mix well .
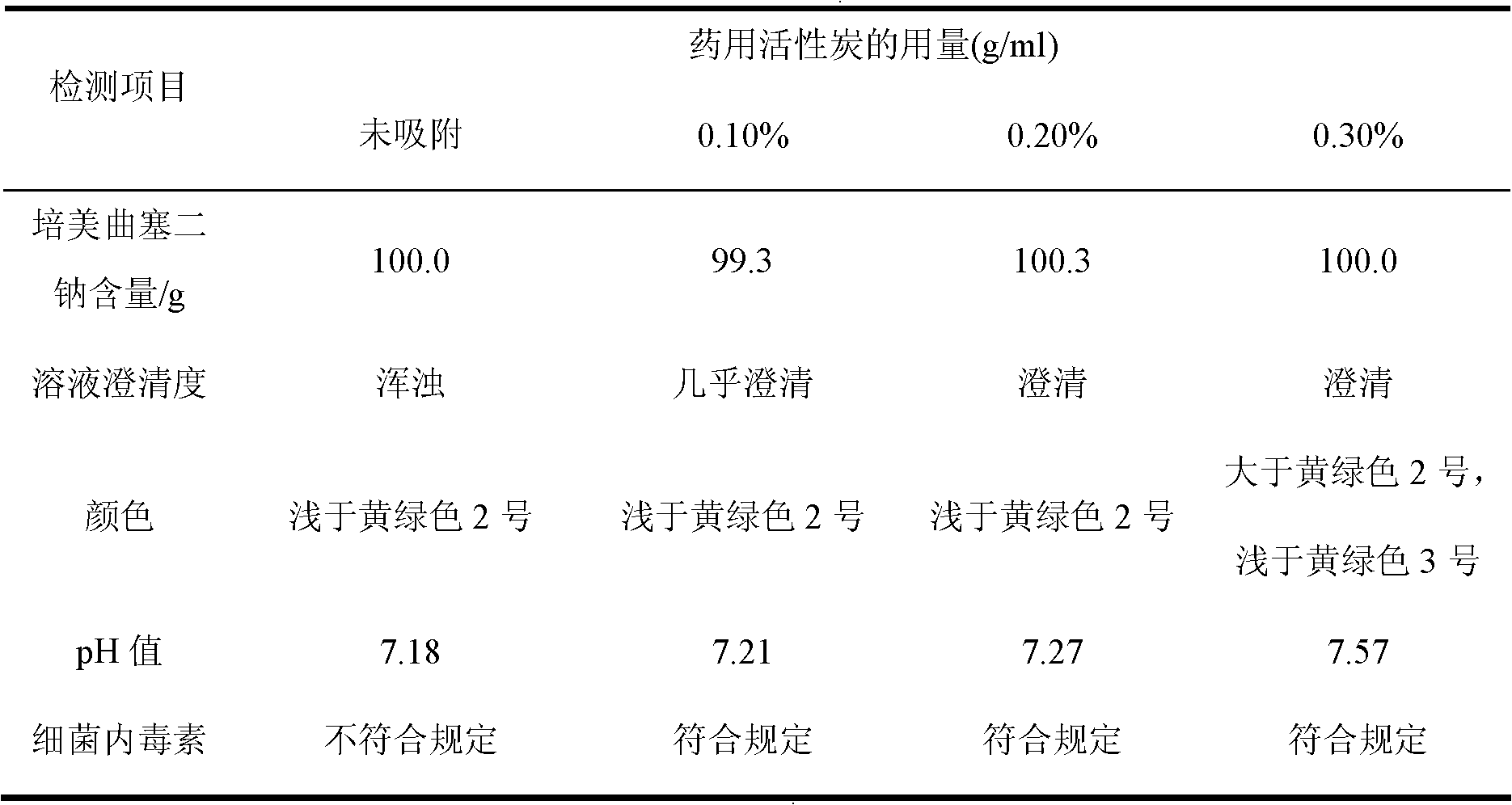
[0048] Add 0.20% (g / ml) of medicinal activated carbon to the clear solution, stir and absorb for 30 minutes, filter with a 0.45 μm microporous membrane for decarburization, filter with a 0.2 μm primary sterile filter and 0.2 μm secondary Terminal sterilization and filtration, the obtained filtrate is put into the liquid medicine bottle for filling.
[0049] The filtrate is subpackaged, half-stoppered, and put into a freeze dryer for freeze-drying. The freeze-drying is divided into three stages: pre-freezing, primary drying and secondary drying;...
Embodiment 2
[0055] Add 3060ml of water for injection into the liquid preparation tank, the temperature of the liquid preparation tank is 0°C, then add 80g of pemetrexed disodium, stir to dissolve. Then add 160g of mannitol, stir to make the liquid into a clear solution; use 0.1mol / L hydrochloric acid solution and / or 0.1mol / L sodium hydroxide solution to adjust the pH value of the liquid to 7.0, add 540ml of water for injection, and mix well .
[0056] Add 0.20% (g / ml) of medicinal activated carbon to the clear solution, stir and absorb for 30 minutes, filter with a 0.45 μm microporous membrane for decarburization, filter with a 0.2 μm primary sterile filter and 0.2 μm secondary Terminal sterilization and filtration, the obtained filtrate is put into the liquid medicine bottle for filling.
[0057] The filtrate is subpackaged, half-stoppered, and put into a freeze dryer for freeze-drying. The freeze-drying is divided into three stages: pre-freezing, primary drying and secondary drying;
...
Embodiment 3
[0063] Add 2652ml of water for injection into the liquid preparation tank, the temperature of the liquid preparation tank is 10°C, then add 80g of pemetrexed disodium, stir to dissolve. Then add 128g of mannitol, stir to make the liquid into a clear solution; use 0.1mol / L hydrochloric acid solution and / or 0.1mol / L sodium hydroxide solution to adjust the pH value of the liquid to 7.54, add 468ml of water for injection, and mix well .
[0064] Add 0.20% (g / ml) of medicinal activated carbon to the clear solution, stir and absorb for 30 minutes, filter with a 0.45 μm microporous membrane for decarburization, filter with a 0.2 μm primary sterile filter and 0.2 μm secondary Terminal sterilization and filtration, the obtained filtrate is put into the liquid medicine bottle for filling.
[0065] The filtrate is subpackaged, half-stoppered, and put into a freeze dryer for freeze-drying. The freeze-drying is divided into three stages: pre-freezing, primary drying and secondary drying; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com