Chymotrypsin composition freeze-dried powder and preparation method thereof
A technology for chymotrypsin and composition, which is applied in the field of chymotrypsin composition freeze-dried powder and its preparation, can solve the problems of chymotrypsin susceptible bacteria, unstable aqueous solution, poor thermal stability of chymotrypsin, etc. Stable, uniform color effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] A chymotrypsin composition freeze-dried powder, the formula is: chymotrypsin 40 million units; dextran 20:40g; sorbitol 2g.
[0063] The average porosity of the freeze-dried powder of the chymotrypsin composition is 85%.
[0064] The preparation method of described chymotrypsin composition freeze-dried powder comprises the steps:
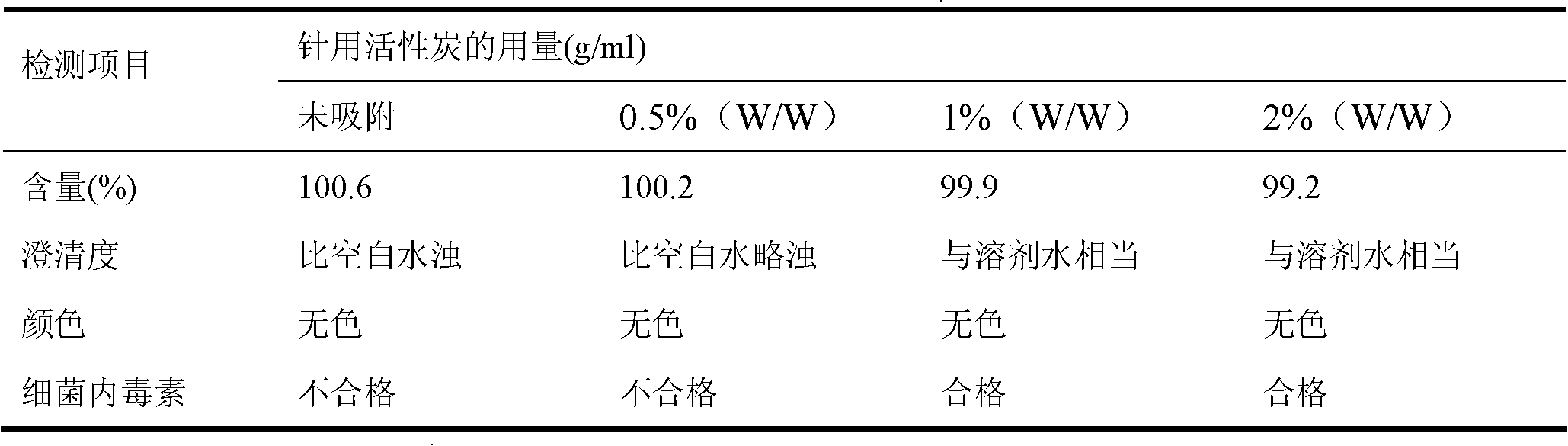
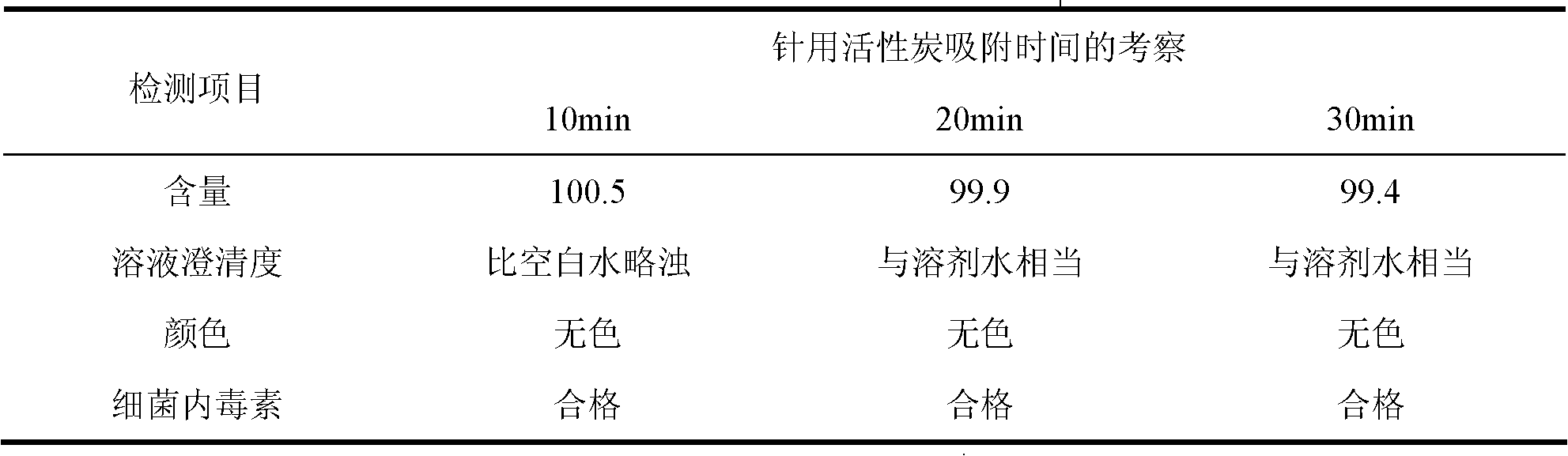
[0065] 1. Prepare the solution: prepare 6% dextran 20 solution with water for injection at a temperature of 10°C, add activated carbon for needles, stir, heat to 90°C for 30 minutes, and then decarbonize and filter through titanium rods; among them, add the mass percentage of activated carbon for needles The concentration is 2%;
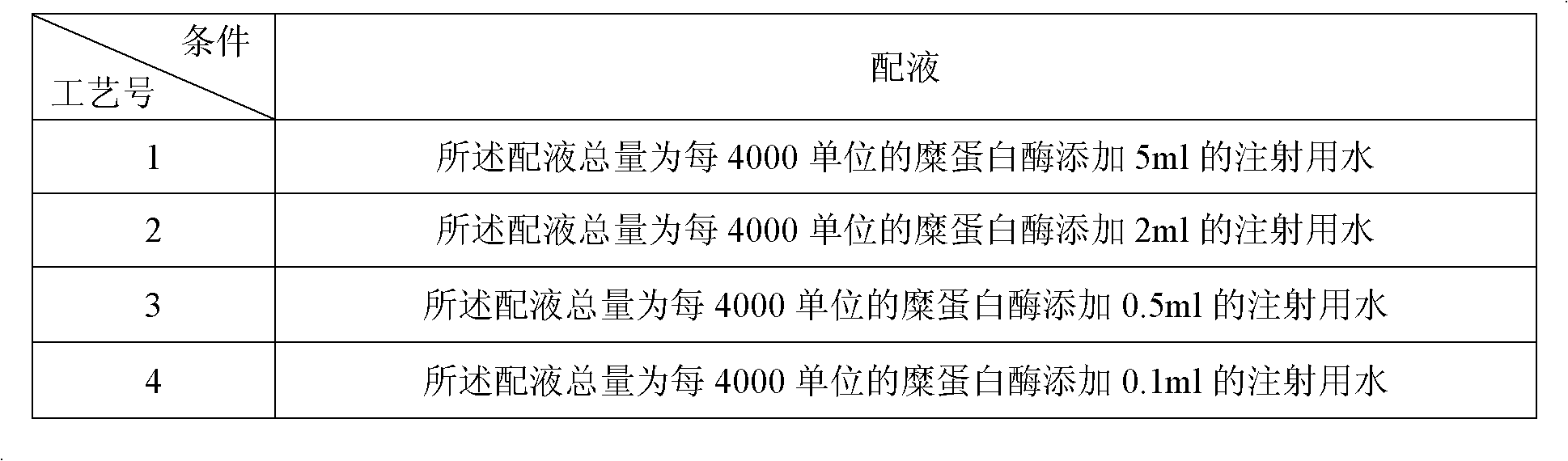
[0066] 2. Add water for injection with a temperature of 5°C and a volume of 60% of the total volume of the liquid in the liquid mixing tank, stir, add 6% dextran 20 solution, 20% sorbitol injection, and chymotrypsin in sequence, and stir until the chymotrypsin is completely Dissolve, add injection to the total amo...
Embodiment 2
[0076] A chymotrypsin composition freeze-dried powder, the formula is: chymotrypsin 40 million units; dextran 20:48g; sorbitol 6g.
[0077] The average porosity of the freeze-dried powder of the chymotrypsin composition is 95%.
[0078] The preparation method of described chymotrypsin composition freeze-dried powder comprises the steps:
[0079] 1. Prepare the solution: prepare 6% dextran 20 solution with water for injection at a temperature of 10°C, add activated carbon for needles, stir, heat to 90°C for 25 minutes, and then decarbonize and filter through titanium rods; among them, add the mass percentage of activated carbon for needles The concentration is 2%;
[0080] 2. Add water for injection with a temperature of 5°C and a volume of 60% of the total volume of the liquid in the liquid preparation tank, stir, then add 6% dextran 20 solution, 20% sorbitol injection, and chymotrypsin, and stir until the chymotrypsin is completely Dissolve and add injection to the total am...
Embodiment 3
[0090] A chymotrypsin composition freeze-dried powder, the formula is: chymotrypsin 40 million units; dextran 20: 56g: sorbitol 4g.
[0091] The average porosity of the freeze-dried powder of the chymotrypsin composition is 90-98%.
[0092] The preparation method of described chymotrypsin composition freeze-dried powder comprises the steps:
[0093] 1. Prepare the solution: prepare 6% dextran 20 solution with water for injection at a temperature of 10°C, add activated carbon for needles, stir, heat to 100°C for 15 minutes, filter and decarbonize; add activated carbon for needles with a concentration of 2% by mass;
[0094] 2. Add water for injection with a temperature of 10°C and a volume of 70% of the total volume of the liquid in the liquid preparation tank, stir, add 6% dextran 20 solution, 20% sorbitol injection, and chymotrypsin in turn, and stir until the chymotrypsin is completely Dissolve and add injection to the total amount of liquid; the total amount of liquid is t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com