Method for preparing gemcitabine hydrochloride lyophilized powder
A technology for gemcitabine hydrochloride and freeze-dried powder injection, which is applied in the field of preparing gemcitabine hydrochloride freeze-dried powder for injection, can solve problems such as poor clarity, and achieve the effects of low drying temperature, simple operation and low content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
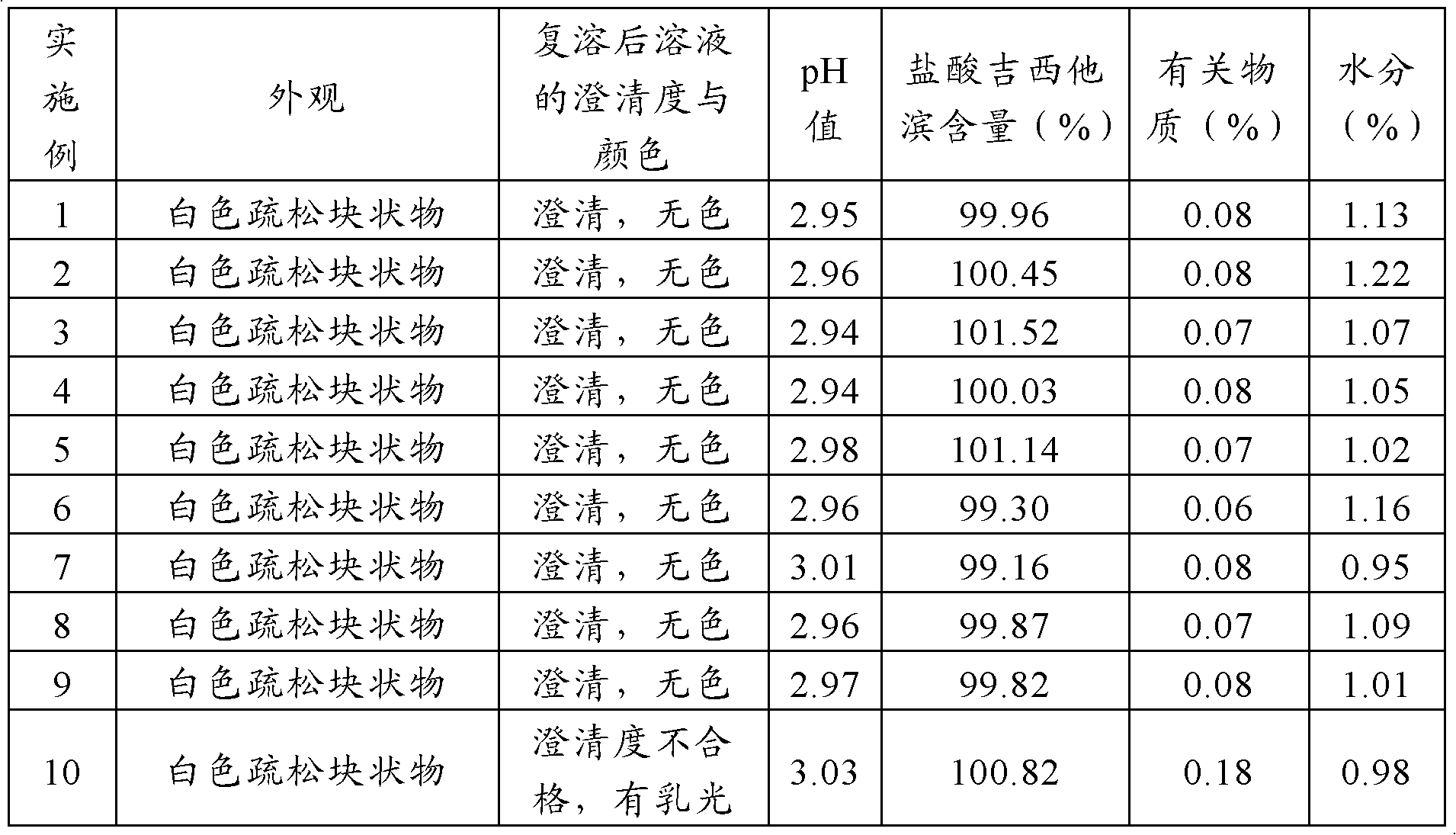
[0036] Dosing: Weigh 228g of gemcitabine hydrochloride, add 4.5L of water for injection, stir to dissolve, then add 200g of mannitol and 12.5g of sodium acetate, stir to dissolve completely, then add water for injection to 5L. Add 2.5g of activated carbon, stir and let stand for 15 minutes, filter, fill with 5mL / bottle, stopper halfway, and send it to a freeze-drying box.
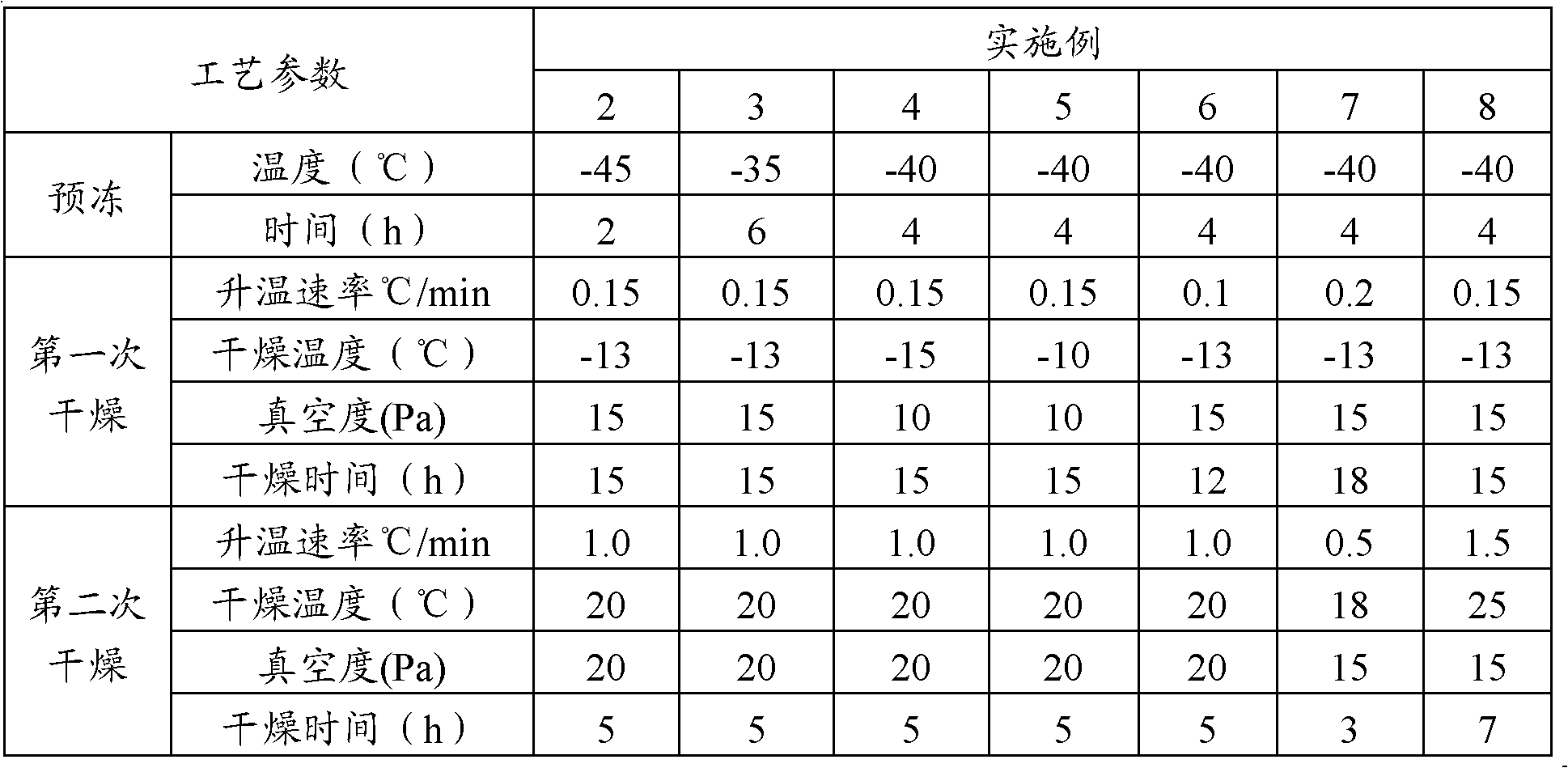
[0037] Freeze-drying: lower the temperature of the freeze-drying box to -40°C and keep it warm for 4 hours. Turn on the vacuum pump, and after the vacuum degree in the drying oven is 20-30Pa, start to heat up and dry at a rate of 0.15°C / min, so that the product temperature rises to -13°C, control the vacuum degree at 15-30Pa, and dry for 15 hours. Then, the temperature was raised at a rate of 1.0°C / min, and after the temperature of the product was raised to 20°C, the vacuum degree was controlled at 15-30Pa for 5 hours. The freeze-dried product is stuffed, out of the box, covered with an aluminum cover, and...
Embodiment 2~8
[0039] The dosing process is the same as in Example 1, and the parameters of the freeze-drying process are shown in Table 1.
[0040] Table 1 embodiment 2~8 lyophilization process
[0041]
Embodiment 9
[0043] The dosing process is the same as in Example 1. In the freeze-drying process, the quick-freezing method is used for pre-freezing, that is, the temperature of the freeze-drying box is lowered to -40° C., and then the samples are put into the freeze-drying process for 4 hours. The rest of the freeze-drying process is the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com