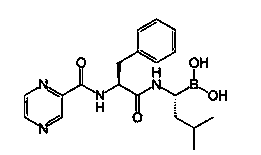
Bortezomib freeze-dried composition and preparation method thereof
A kind of technology of bortezomib and composition, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
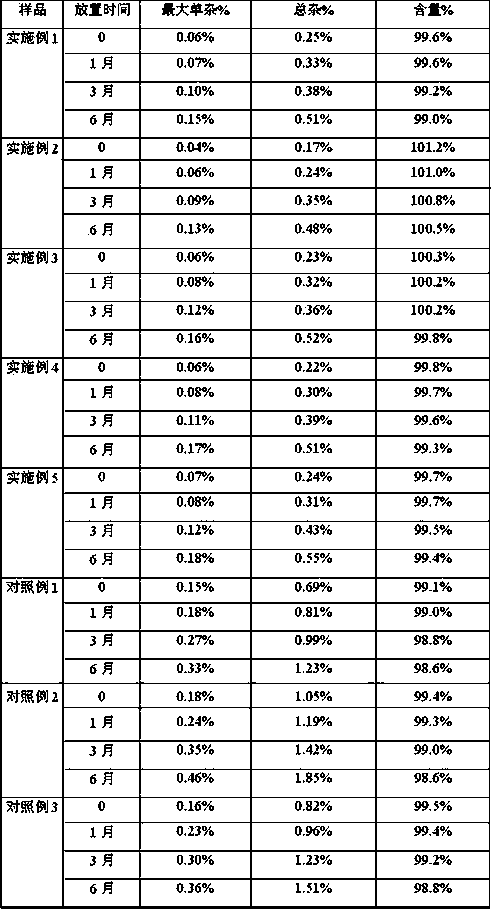
Embodiment 1
[0043] prescription
[0044] Bortezomib 100mg Mannitol 1000mg tert-butanol 20g Water for Injection Dilute to 100ml
[0045] Preparation Process:
[0046]Weigh 20 g of tert-butanol at 40°C that meets the requirements of injection grade, add 100 mg of bortezomib, stir to mix the two evenly, and obtain a tert-butanol solution containing bortezomib, add 1000 mg of mannitol to the above solution, stir and mix Evenly, to obtain a mixed suspension; spray the mixed suspension into liquid nitrogen to form frozen particles, add water for injection to the frozen particles to 90ml, stir at 5°C for about 10 minutes, the main drug is completely dissolved, add water to the full amount, Sampling and detection of intermediates. After the intermediates are qualified, fill them. Cool the filled samples to -40°C for pre-freezing and keep them for 1 hour; raise the temperature to -20°C, keep them warm for 2 hours, then cool them down to -45°C and keep them war...
Embodiment 2
[0049] prescription
[0050] Bortezomib 200mg Mannitol 2000mg tert-butanol 40g Water for Injection Dilute to 100ml
[0051] Preparation Process:
[0052] Weigh 42g of tert-butanol at 25°C that meets the requirements of injection grade, add bortezomib 200mg, stir to mix the two evenly, and obtain a tert-butanol solution containing bortezomib, add 2000mg of mannitol to the above solution, stir and mix Evenly, a mixed suspension is obtained; spray the mixed suspension into liquid nitrogen to form frozen particles, add water for injection to the frozen particles to 90ml, stir at 25°C for about 8 minutes, the main drug is completely dissolved, add water to the full amount, Sampling and detection of intermediates. After the intermediates are qualified, fill them. Cool the filled samples to -45°C for pre-freezing and keep them for 3 hours; heat them up to -25°C, keep them warm for 3 hours, then cool them down to -40°C and keep them warm for 3 hou...
Embodiment 3
[0056] prescription
[0057] Bortezomib 175mg Mannitol 1750mg tert-butanol 33g Water for Injection Dilute to 100ml
[0058] Preparation Process:
[0059] Weigh 33g of tert-butanol that meets the requirements of injection grade at 30°C, add 175mg of bortezomib, and mix well to obtain a tert-butanol solution containing bortezomib, add 150mgg mannitol to the above solution, stir and mix evenly to obtain a mixed Suspension: Spray the mixed suspension into liquid nitrogen to form frozen particles, add water for injection to 90ml of frozen particles, stir at 25°C for about 10 minutes, the main drug is completely dissolved, add water to the full amount, and take a sample to detect the intermediate , after the intermediate is qualified, it is filled, the filled sample is cooled to -50°C for pre-freezing, and kept for 2 hours; the temperature is raised to -20°C, kept for 3 hours, and then cooled to -50°C for 2 hours; open the cold trap, open Vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com