Spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection and preparation method of spectinomycin hydrochloride and lincomycin hydrochloride freeze-dried powder injection
A technology of lincomycin hydrochloride and spectinomycin hydrochloride, which is applied in freeze-drying transportation, powder transportation, pharmaceutical formulations, etc., can solve the problems of poor drug stability and high production costs, achieve drug stability, keep pH unchanged, and reduce The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
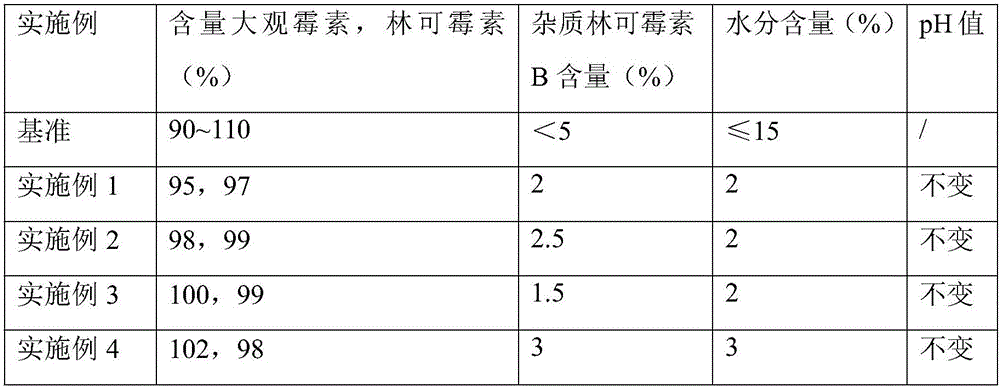
Embodiment 1
[0025] Embodiment 1 (taking spectinomycin hydrochloride as mass ratio benchmark),
[0026] component name Material name mass ratio spectinomycin hydrochloride spectinomycin hydrochloride 1 Lincomycin Hydrochloride Lincomycin Hydrochloride 1 freeze-dried proppant lactose 1 Antioxidants sodium bisulfite 0.1
[0027] Wherein, the solid content in the solution after constant volume in step (2) is 15%, the antioxidant content in the solution after constant volume in step (2) is 0.5wt%, and the volume of water for injection in step (1) is controlled at 5mL~20mL.
Embodiment 2
[0028] Embodiment 2 (taking spectinomycin hydrochloride as mass ratio benchmark),
[0029] component name Material name mass ratio spectinomycin hydrochloride spectinomycin hydrochloride 4 Lincomycin Hydrochloride Lincomycin Hydrochloride 4 freeze-dried proppant lactose 10 Antioxidants sodium bisulfite 1
[0030] Wherein, the solid content in the solution after constant volume in step (2) is 15%, the antioxidant content in the solution after constant volume in step (2) is 0.5wt%, and the volume of water for injection in step (1) is controlled at 5mL~20mL.
Embodiment 3
[0031] Embodiment 3 (taking spectinomycin hydrochloride as mass ratio benchmark),
[0032] component name Material name mass ratio spectinomycin hydrochloride spectinomycin hydrochloride 4 Lincomycin Hydrochloride Lincomycin Hydrochloride 2 freeze-dried proppant lactose 4 Antioxidants sodium bisulfite 0.5
[0033] Wherein, the solid content in the solution after constant volume in step (2) is 15%, the antioxidant content in the solution after constant volume in step (2) is 0.5wt%, and the volume of water for injection in step (1) is controlled at 5mL~20mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com