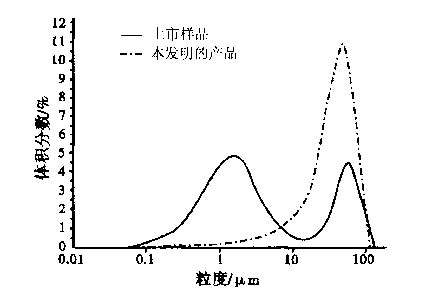
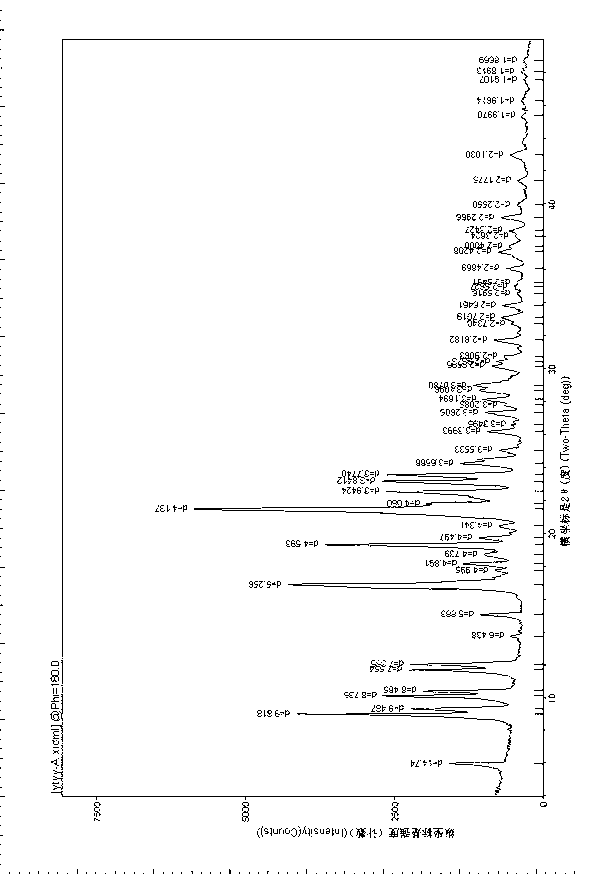
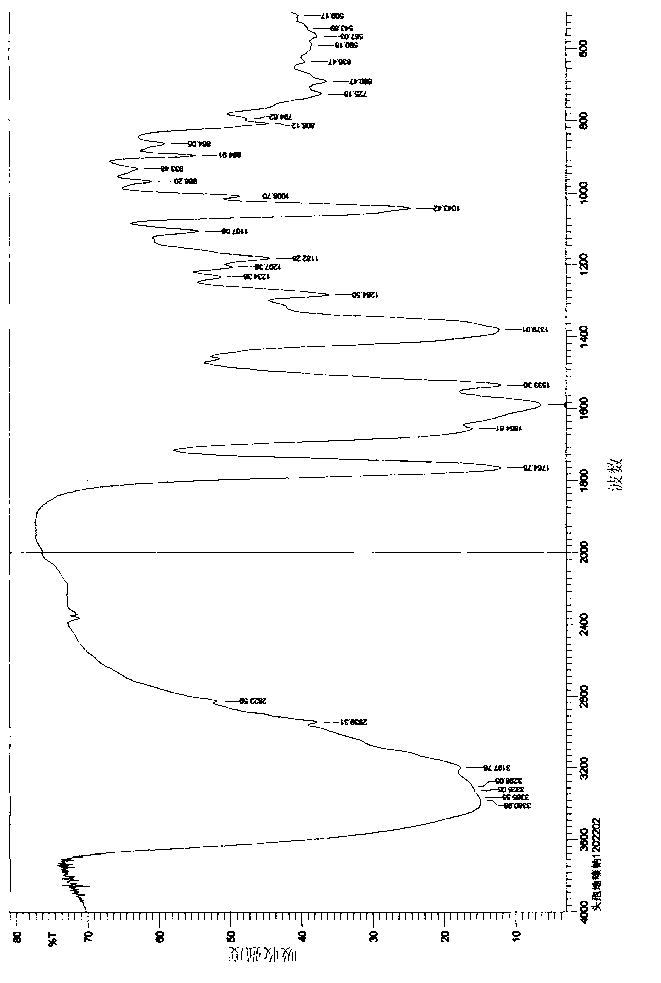
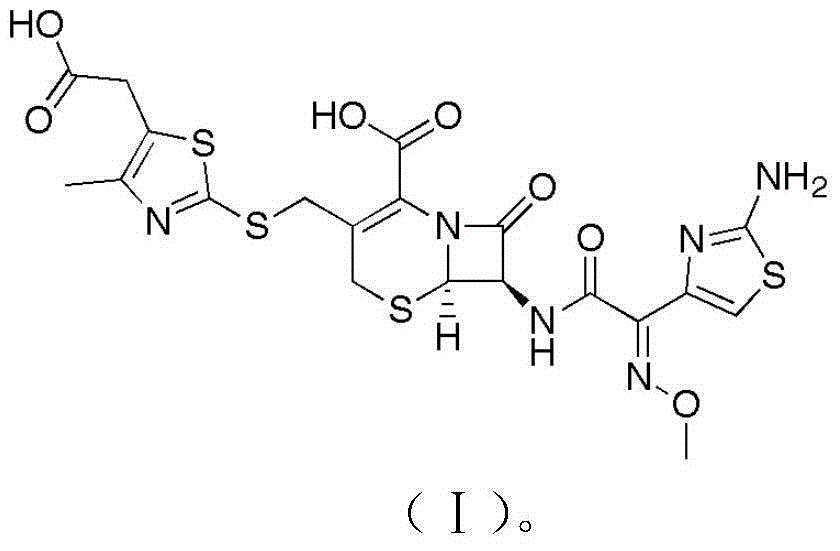
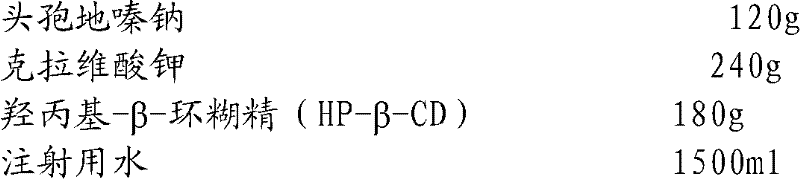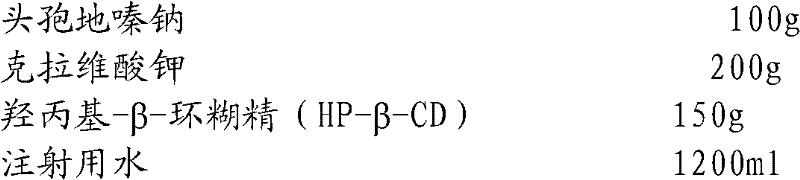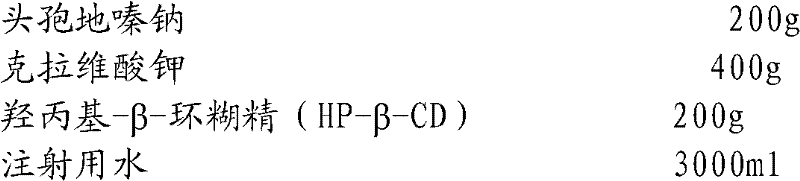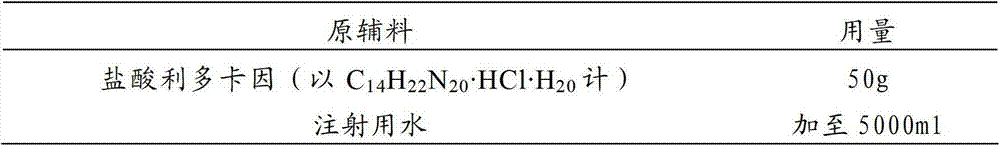Patents
Literature
55 results about "Cefodizime Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
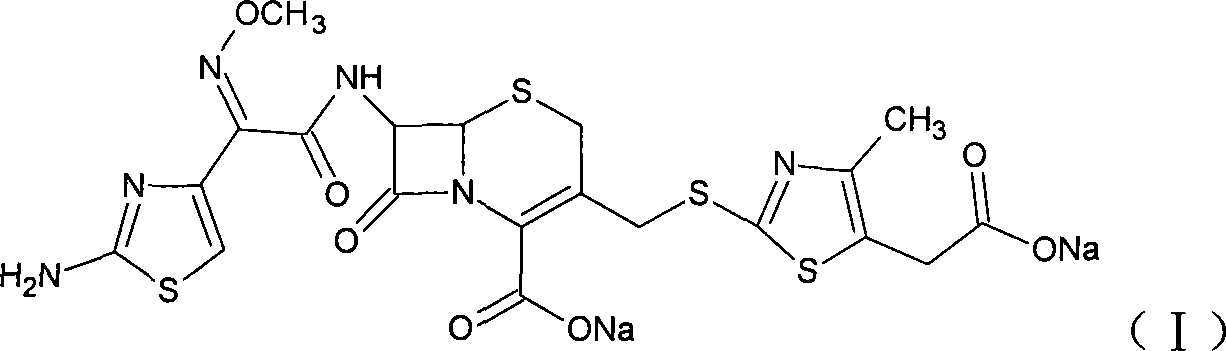
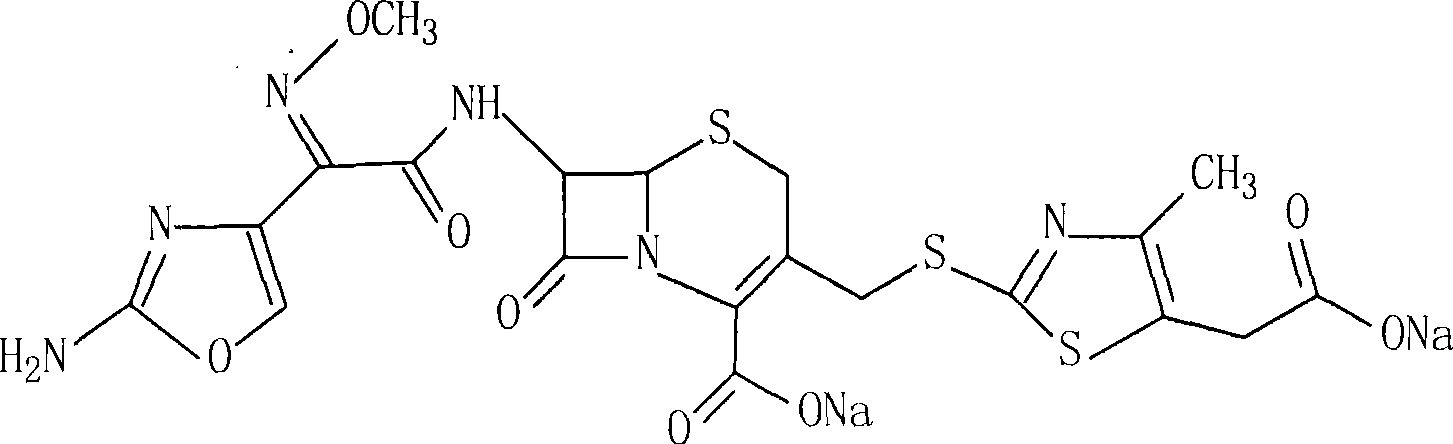
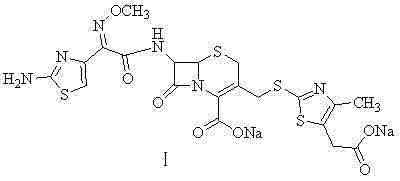
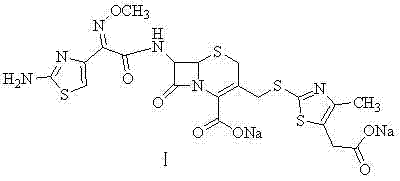
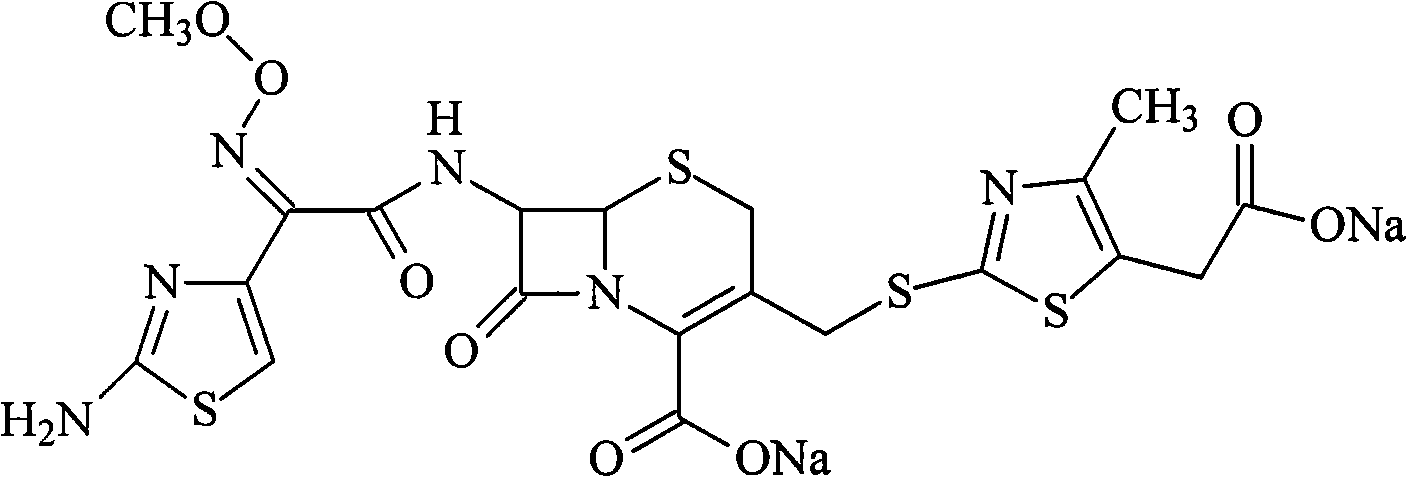
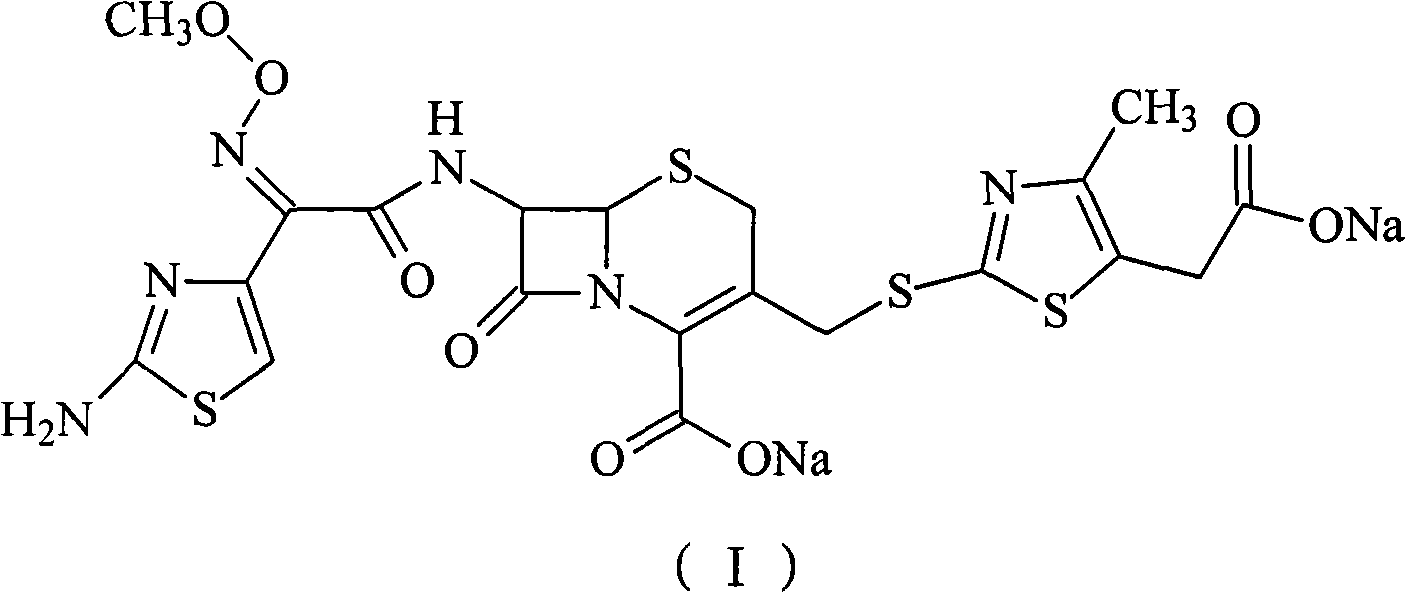
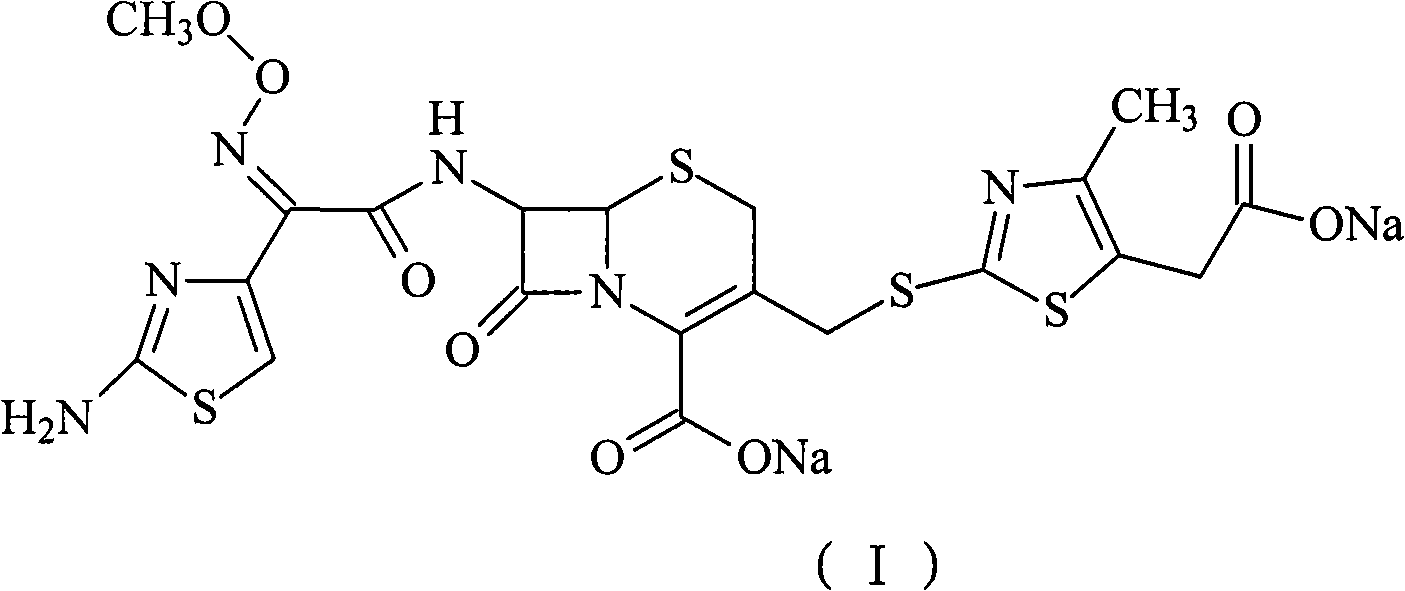
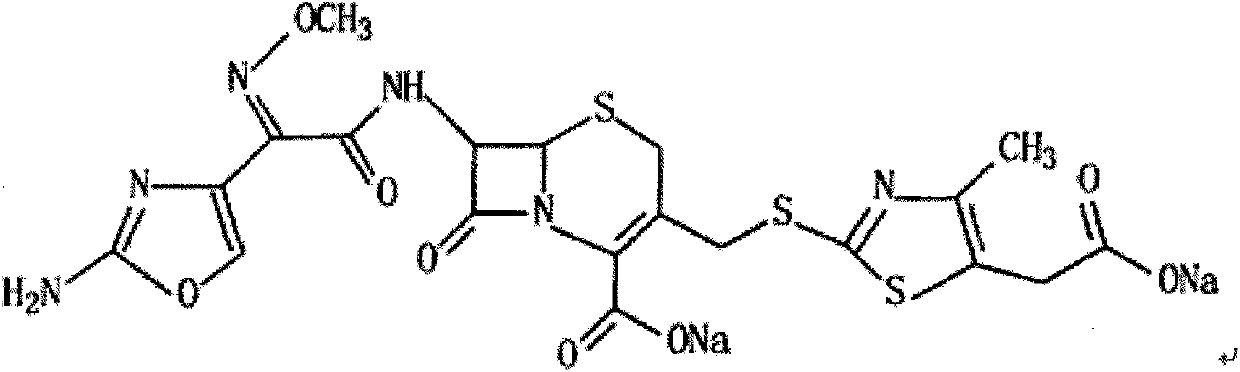
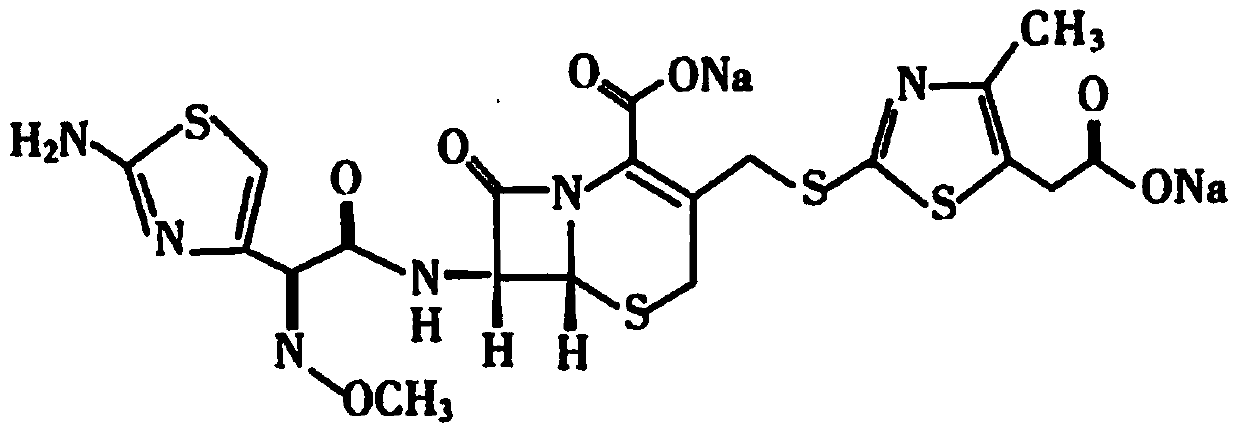
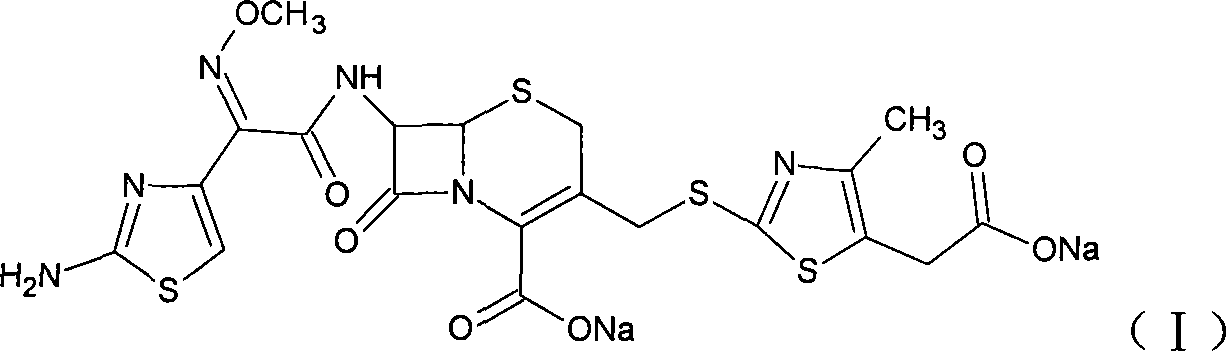
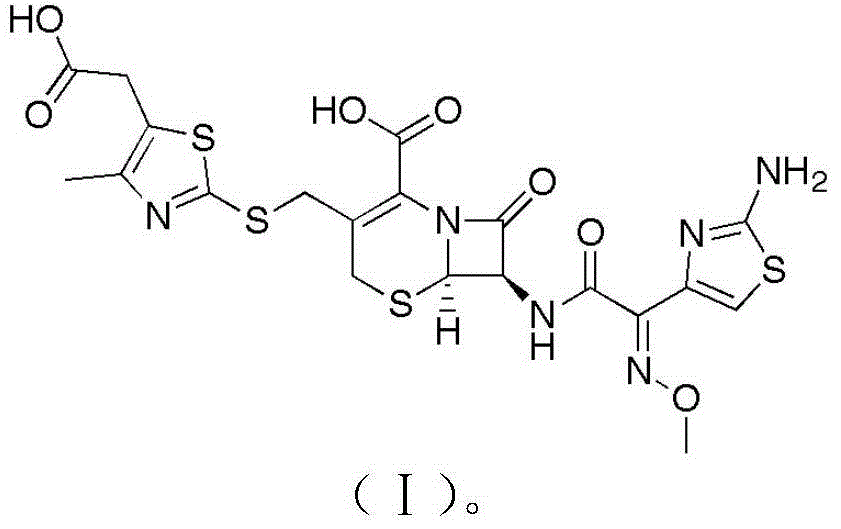
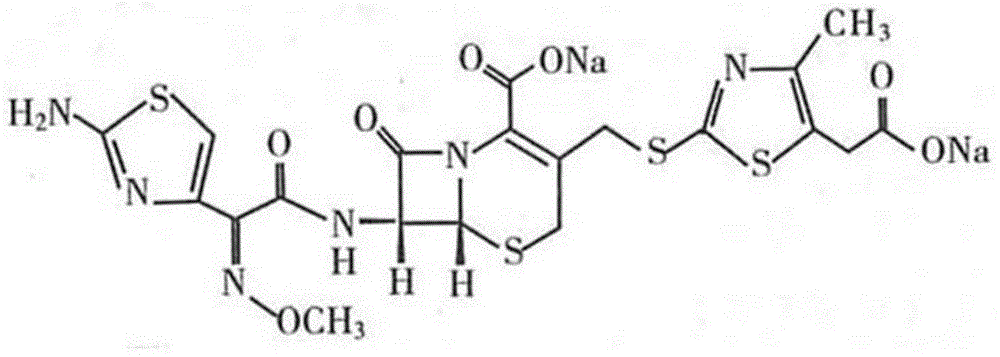
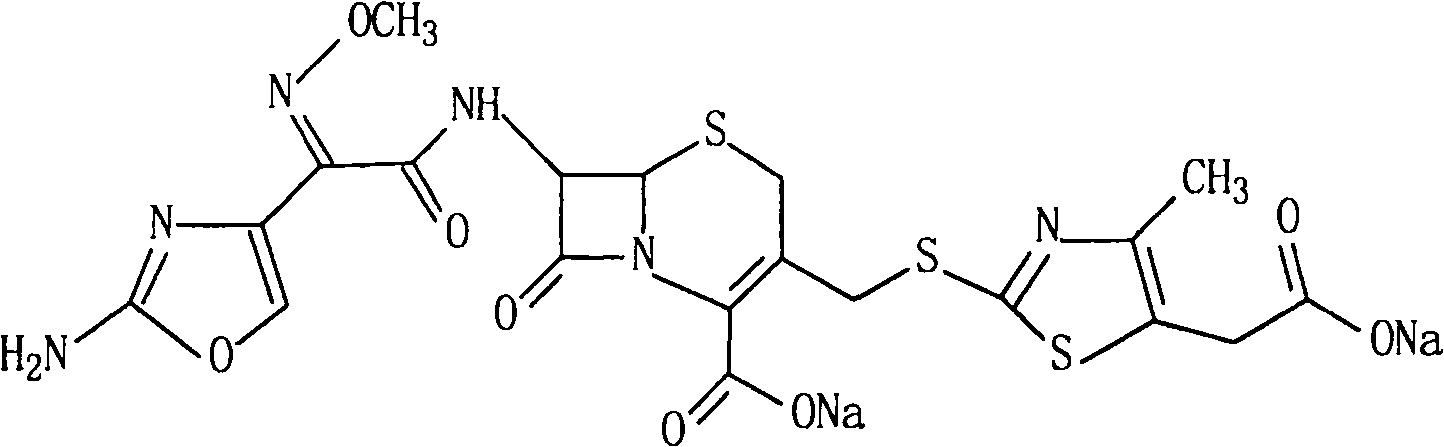
The sodium salt form of cefodizime, a third-generation, aminothiazolyl cephalosporin for parenteral use. Cefodizime has broad-spectrum activity and is stable to most beta-lactamases.
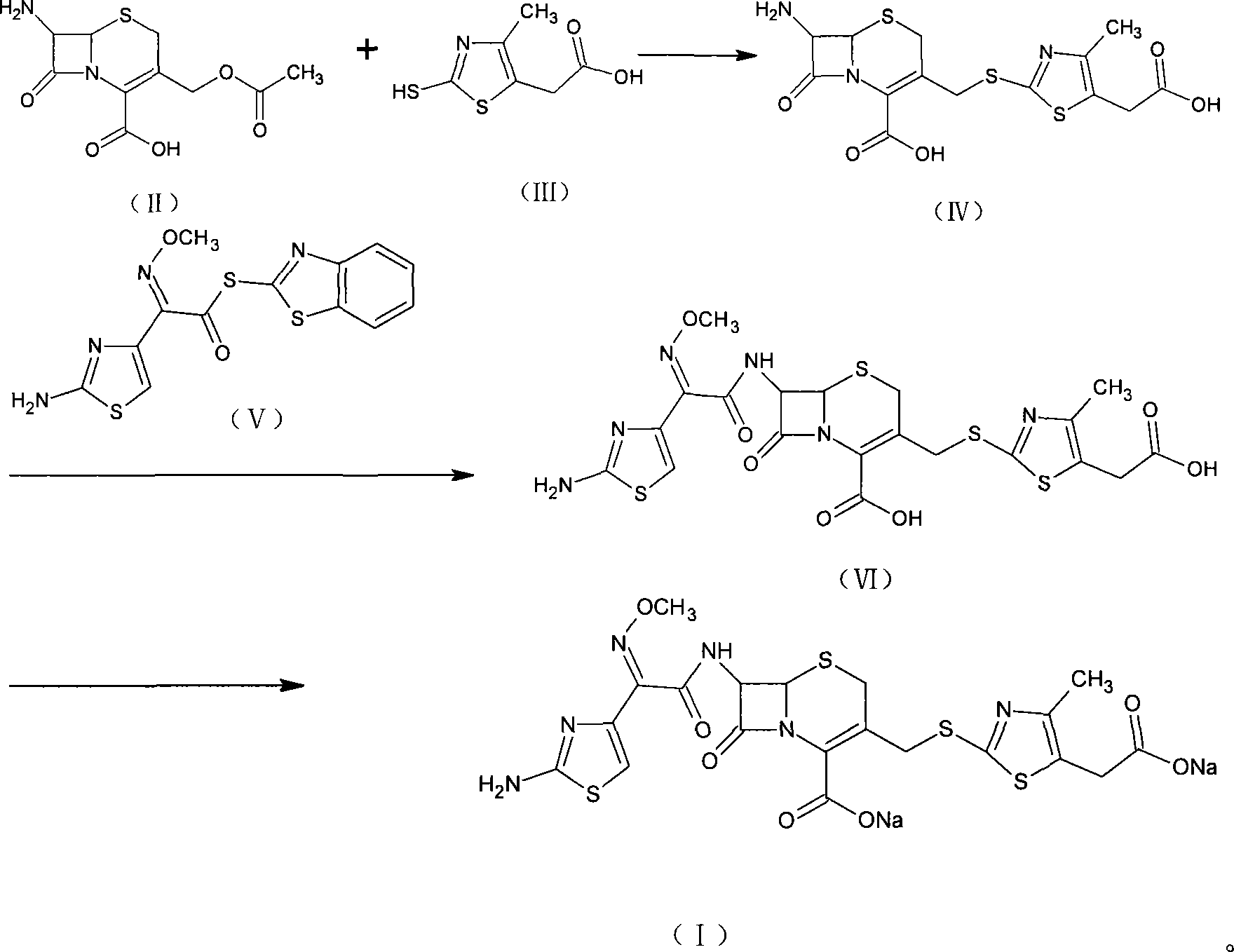
Method for preparing cefodizime sodium
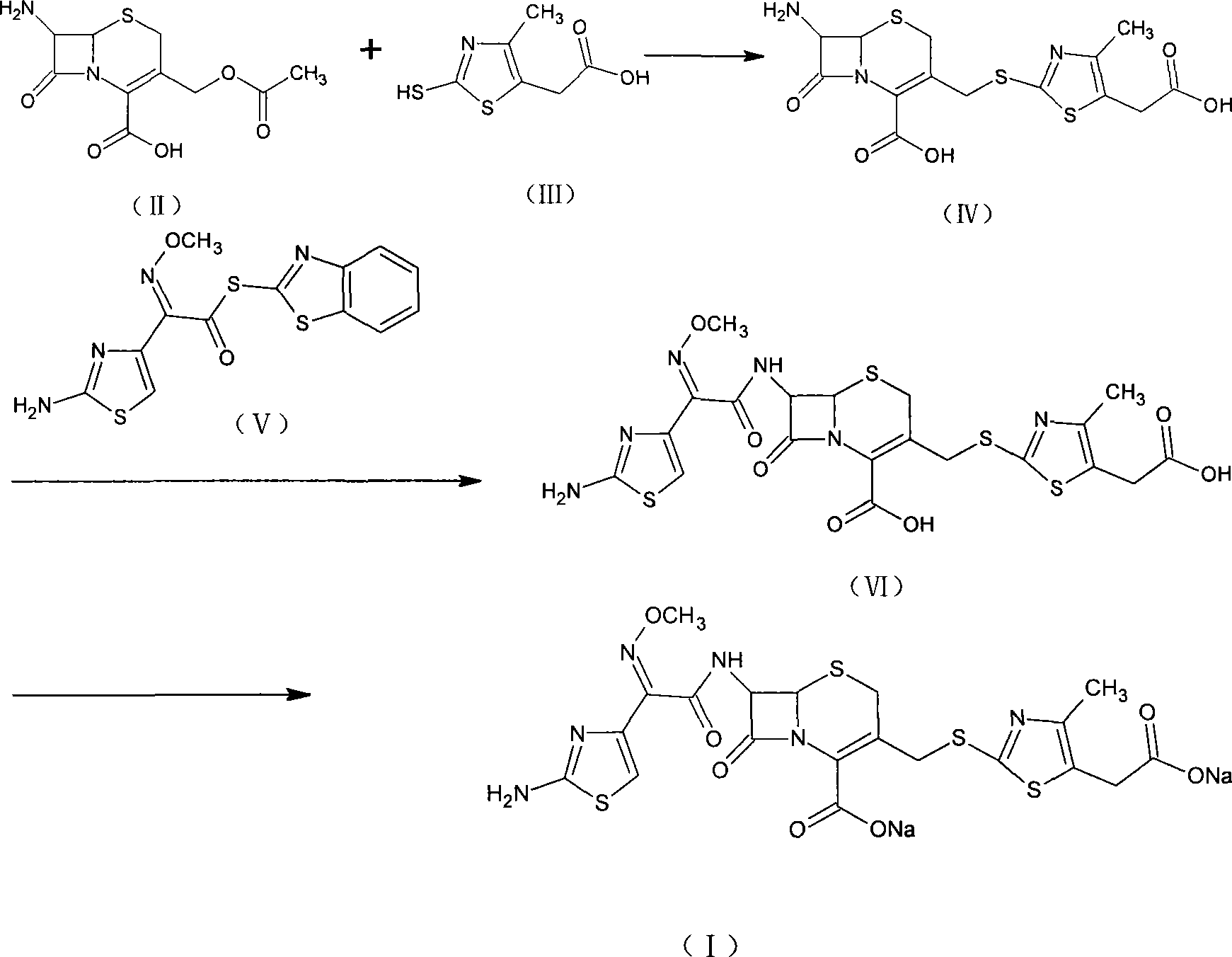
ActiveCN101239985AEasy to operateLow costAntibacterial agentsOrganic active ingredientsCefodizime SodiumSolvent
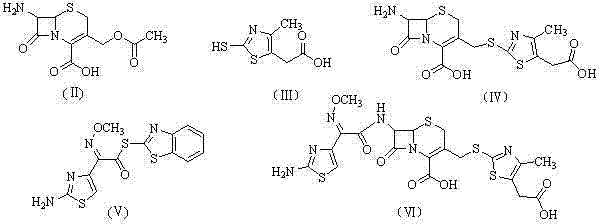
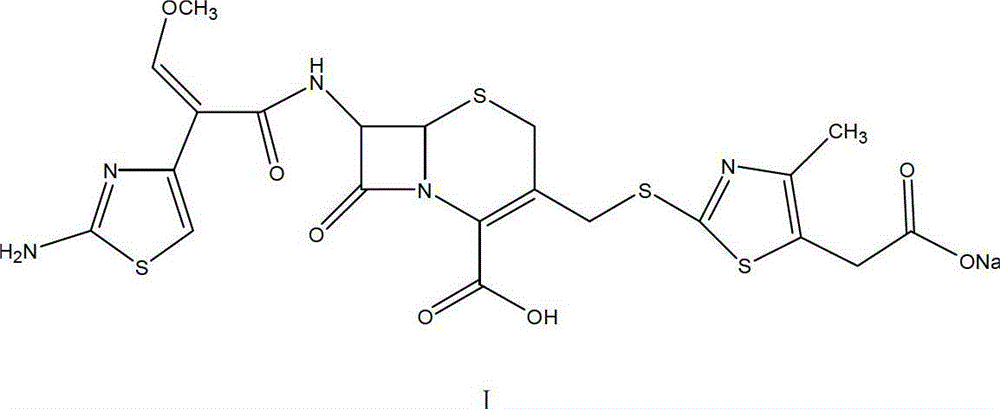
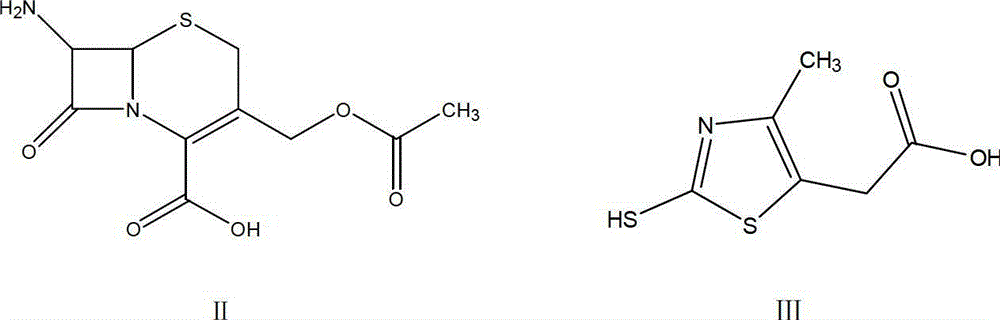
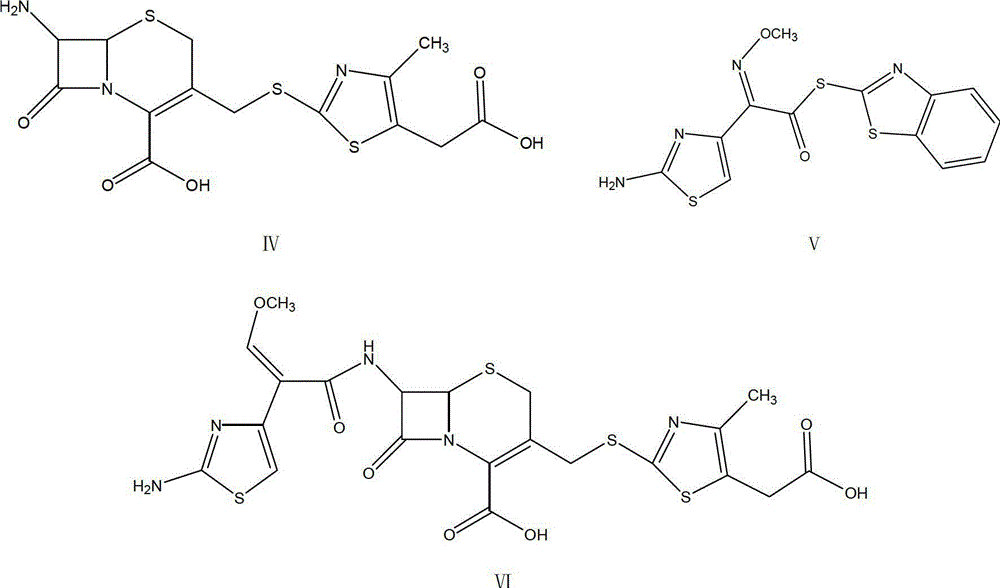
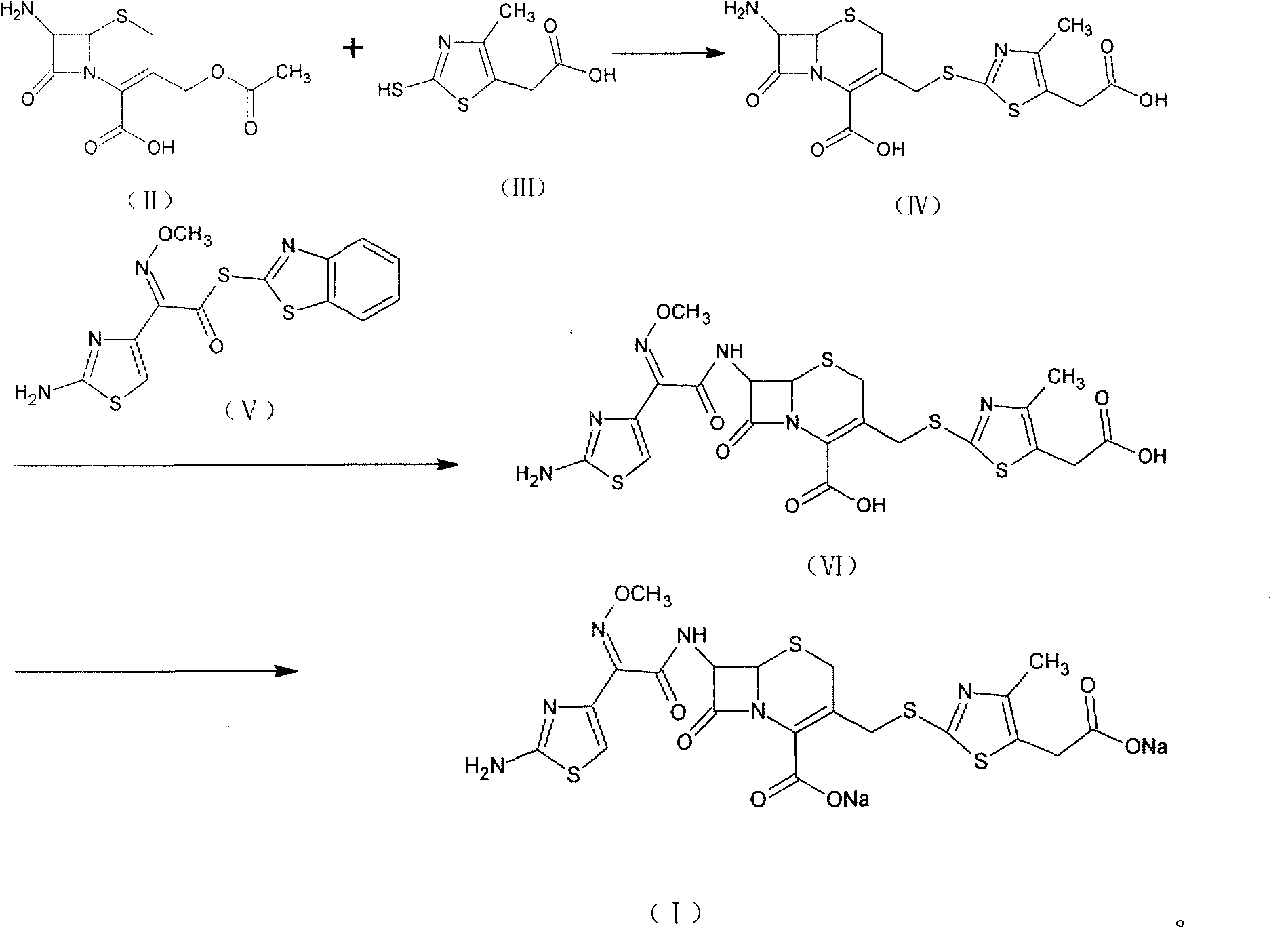
The invention relates to preparation of a cefodizime sodium. The preparation includes reacting compound of formula (II) and compound of formula (III) in presence of acid catalyst to obtain compound of formula (IN); acidylating compound of formula (IN) and compound of formula (V) to obtain compound of formula (VI) in mixed solution; generating compound of formula (I) by compound of formula (VI) in presence of salt forming agents in mixed solution. The invention is easy to operate, high in yield, and low in cost.
Owner:QILU ANTIBIOTICS PHARMA
Cefodizime sodium proliposome preparation and preparation method thereof
InactiveCN101584664AUnexpected effectNo toxicityAntibacterial agentsOrganic active ingredientsCefodizime SodiumCholesterol
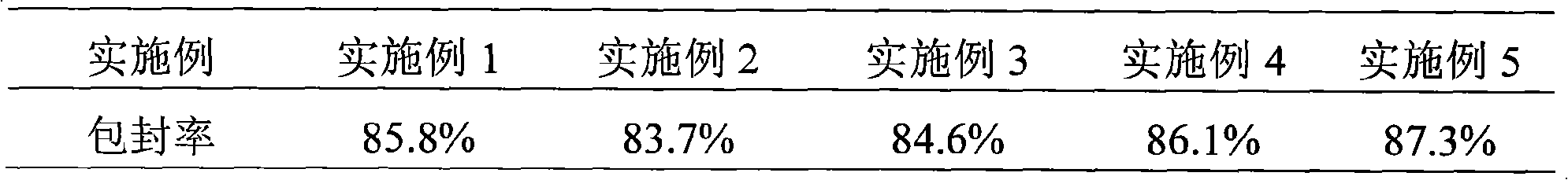
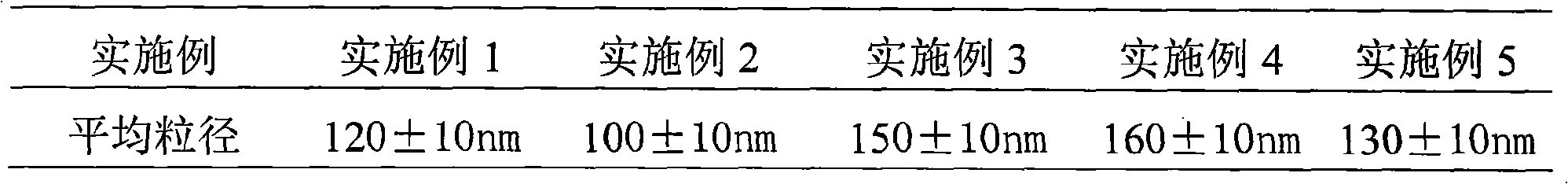
The invention relates to a cefodizime sodium proliposome preparation and a preparation method thereof. The proliposome preparation comprises cefodizime sodium, egg yolk lecithin, cholesterol, antioxidant and supporting agent, wherein the cefodizime sodium proliposome preparation comprises the following components by weight portion: 1 to 20 portions of the cefodizime sodium, 5 to 50 portions of the egg yolk lecithin, 3 to 30 portions of the cholesterol, 0.5 to 20 portions of the antioxidant and 3 to 50 portions of the supporting agent.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefodizime sodium compound and method for synthesizing the same
InactiveCN101486720AEasy to operateLow costAntibacterial agentsOrganic chemistrySodium acetateAcetic acid
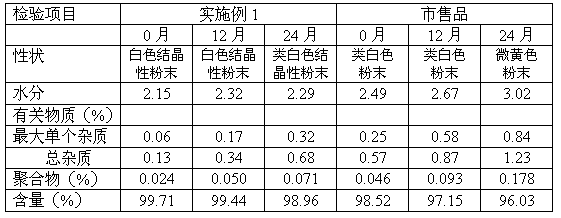
The invention relates to a cefodizime sodium compound and a synthetic method thereof; the synthetic method comprises the step that cefodizime acid reacts with sodium acetate to obtain the cefodizime sodium; the synthetic method also comprises the step that cefotaxime acid reacts with 2-sulfydryl-4-methyl-5-thiazole acetic acid in a hexahydropyridine aqueous solution to produce the cefodizime acid; and the synthetic method of the cefodizime sodium compound has the advantages of simple operation, high yield up to 80 percent and high product purity more than 99.6 percent, thus obtaining surprising technical effects.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefodizime sodium composition and preparation method thereof
InactiveCN102258521AImprove stabilityStable recipe processAntibacterial agentsOrganic active ingredientsCefodizime SodiumFreeze-drying
The invention relates to the field of drug synthesis and preparation thereof, relates to a preparation method of an antibacterial drug, in particular to a stable cefodizime sodium composition preparation and a preparation method thereof. The present invention directly dissolves sterile cefodizime sodium in water, adds sterile potassium clavulanate to dissolve it completely, and obtains an aqueous solution of cefodizime sodium / clavulanate potassium, and adds hydroxypropyl-β to the aqueous solution. -Cyclodextrin (HP-β-CD) inclusion, sub-package, and freeze-drying to obtain Cefodizime Sodium / Clavulanate Potassium for Injection. The preparation method provided by the invention is simple, and the cefodizime sodium / clavulanic acid potassium salt is clathrated with hydroxypropyl-β-cyclodextrin, which increases the stability of the sterile cefodizime sodium drug and reduces its toxic and side effects , improve drug availability, and the preparation process is simple, suitable for industrial production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD +1
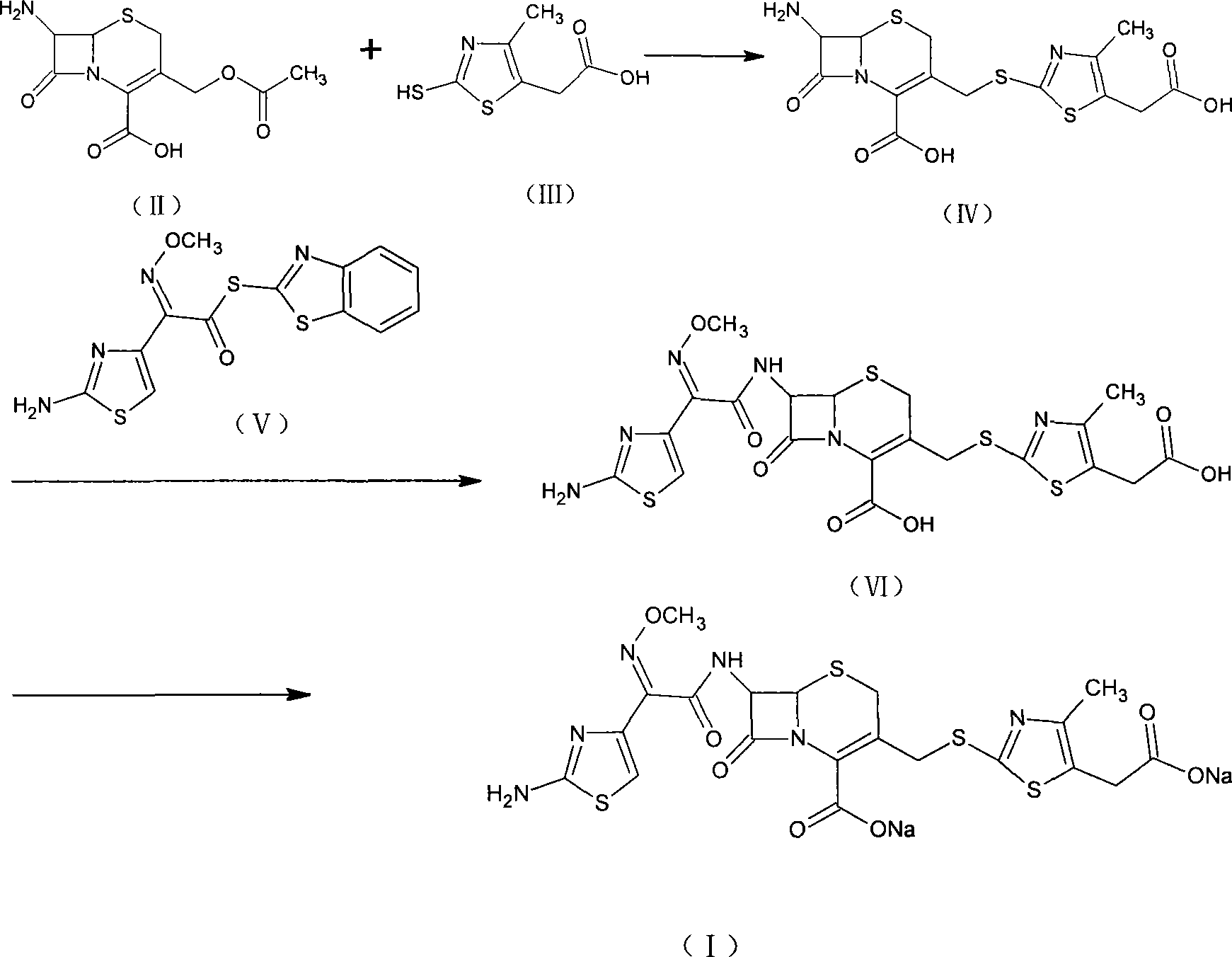
Preparation method of cefodizime sodium
The invention relates to a preparation method of cefodizime sodium. The preparation method comprises the following steps: reacting 7-ACA (7-aminocephalosporanic acid) (II) with 2-sulfydryl-4-methyl-5-thiazoleacetic acid (III) under the alkaline condition so as to obtain an intermediate (IV); acting the intermediate (IV) with AE (active ester) (V) so as to obtain cefodizime acid (VI); and acting cefodizime acid (VI) in the presence of a salifying agent so as to generate the product cefodizime sodium (I). According to the invention, the preparation method of cefodizime sodium has low cost, is simple to operate and is suitable for industrial production, and the yield of cefodizime sodium is up to 63.7%; and the product is white or of white-like color and dose not need to be refined, thus ensuring the quality and yield of the product.
Owner:TIANJIN GREENPINE PHARMA
Cefodizime sodium composition and powder injection
ActiveCN101829119AImprove stabilityStable specific volumeAntibacterial agentsPowder deliveryCefodizime SodiumSodium benzoate
The invention relates to a cefodizime sodium composition, which comprises the following components in percentage by mass: 99.00 to 99.99 percent of cefodizime sodium crystals and 0.01 to 1.00 percent of sodium benzoate. The invention also relates to a preparation containing the cefodizime sodium crystals and the sodium benzoate and a method for preparing the cefodizime sodium crystals. The cefodizime sodium crystals have the advantages of high stability and good liquidity.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Cefodizime sodium compound and novel method thereof
InactiveCN102010432AHigh purityIncrease contentAntibacterial agentsOrganic active ingredientsActivated carbonSide effect
The invention provides a cefodizime sodium compound and a novel method thereof. The purpose of refining purification is achieved by acid-base salifying reaction, activated carbon adsorption and chromatographic column adsorption separation purification, high-purity cefodizime sodium compound is finally obtained, the preparation product quality is improved, the toxic and side effects are reduced, and the safety in the preparation of broad spectrum antibacterial activity medicine usage is ensured; in addition, compared with the prior art, the method is simple, convenient and feasible in process, has low cost, high yield and high product purification, reduces toxic and side effects and is suitable for industrialized production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefodizime sodium hydrate, preparation method thereof and application thereof
InactiveCN101830915AProcess stabilityReduce humidityAntibacterial agentsOrganic chemistryDiseaseCefodizime Sodium
The invention relates to a cefodizime sodium hydrate, a preparation method thereof and application thereof. The cefodizime sodium hydrate has high storage stability, and is suitable to be used in the preparation of medicaments for treating and preventing diseases, caused by Gram-positive or negative bacterial sensitive bacteria, such as diseases in respiratory systems, liver and gall systems and five sense organs of human beings and animals, urinary tract infection, enterocoelia infection, pelvic cavity infection, ichorrhemia, skin and soft tissue infection, bone and joint infection, annexitis, intrauterine infection, parametric connective tissue inflammation, cephalomeningitis, gonorrhoea and the like.
Owner:胡梨芳
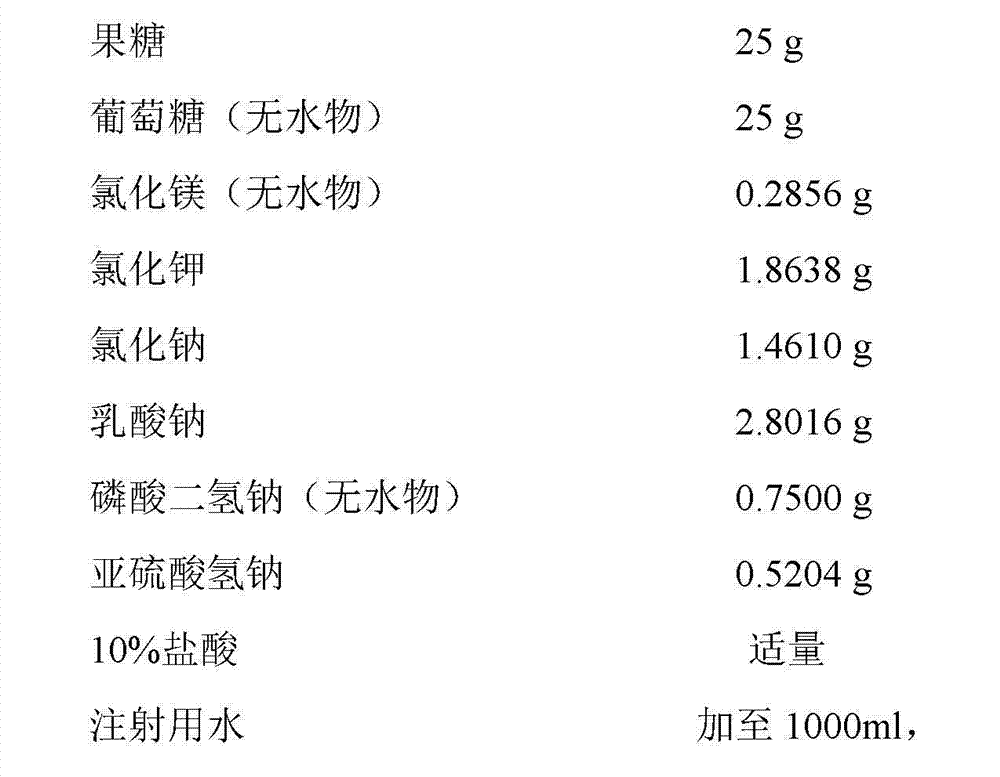
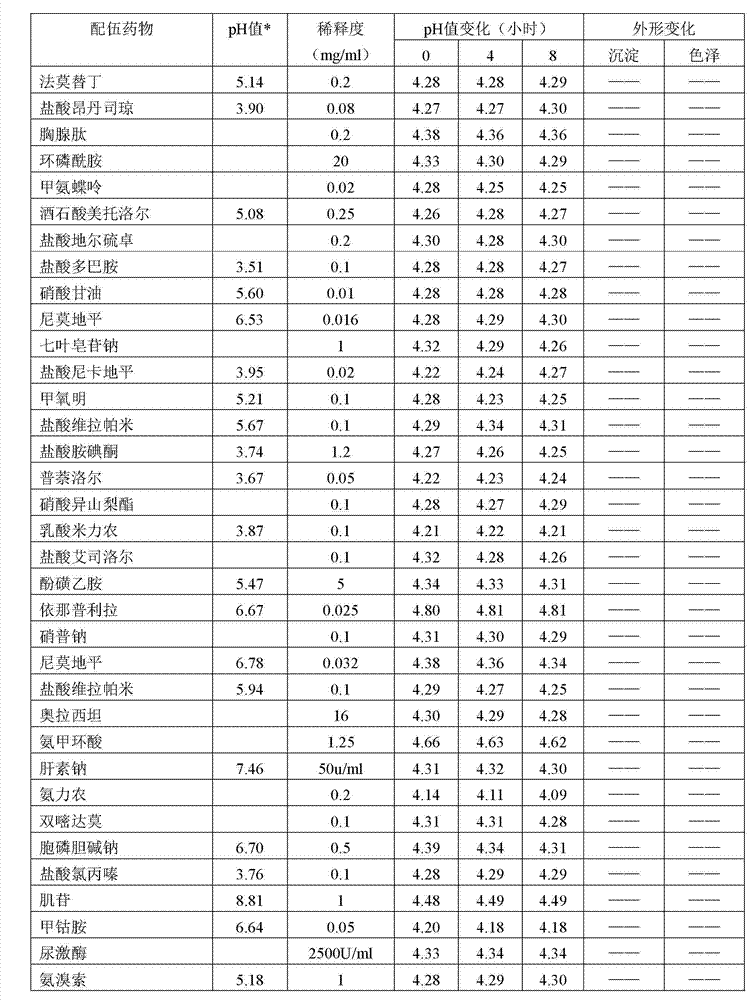
Method for preparing invert sugar and electrolytes injection
ActiveCN102949413AImprove securityImprove applicabilityInorganic phosphorous active ingredientsMetabolism disorderCefodizime SodiumMedicine
The invention belongs to the field of medical technologies, and mainly relates to a method for preparing an invert sugar and electrolytes injection. Specifically, the method disclosed by the invention improves the stability and safety of compatibility from the process source, and the invert sugar and electrolytes injection prepared by using the method disclosed by the invention can be safely compatible with 70 kinds of commonly used clinical medicines such as cefodizime sodium, thereby improving the safety, applicability and convenience of clinical medications.
Owner:HAISCO PHARMA GRP INC
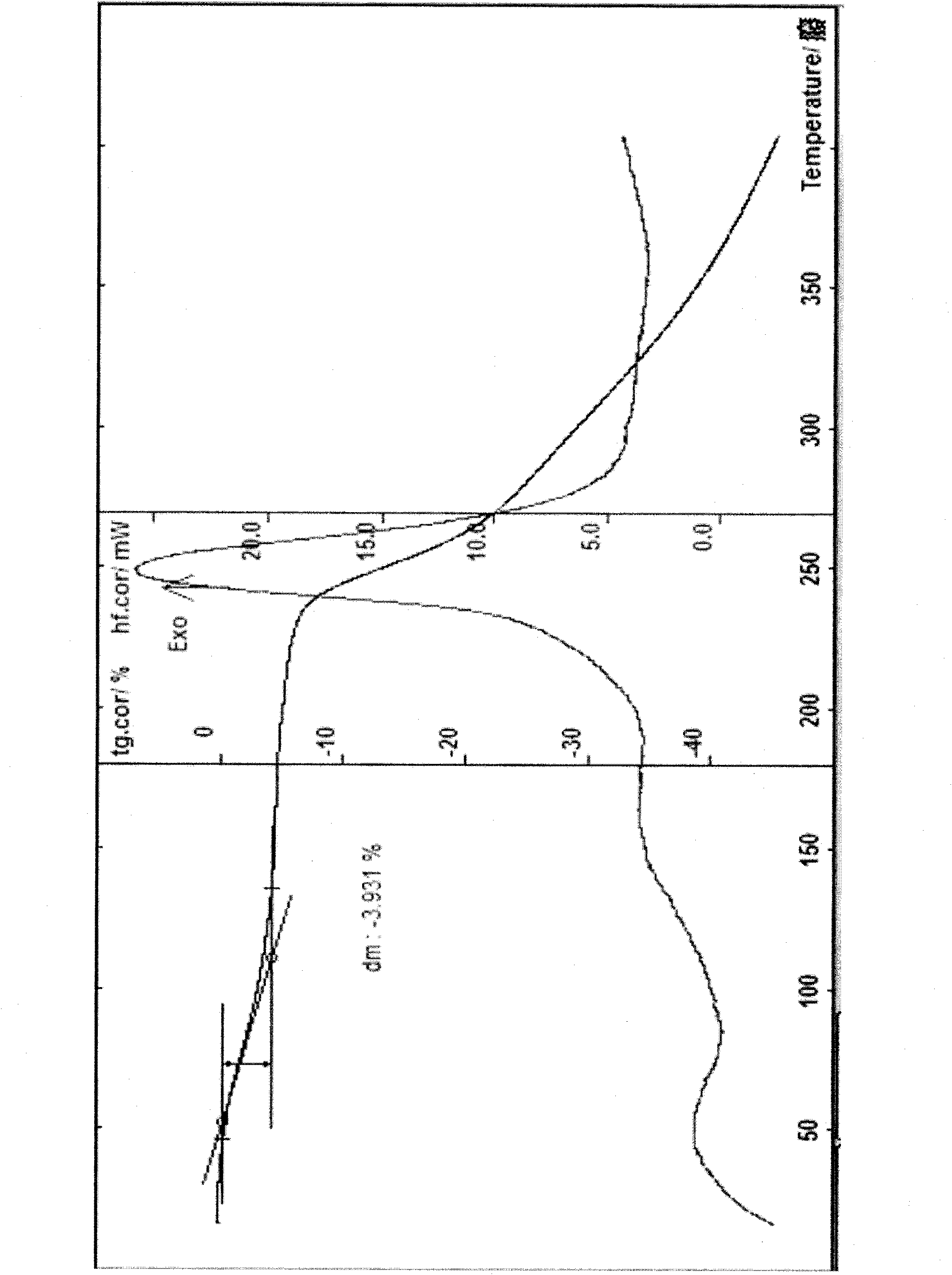
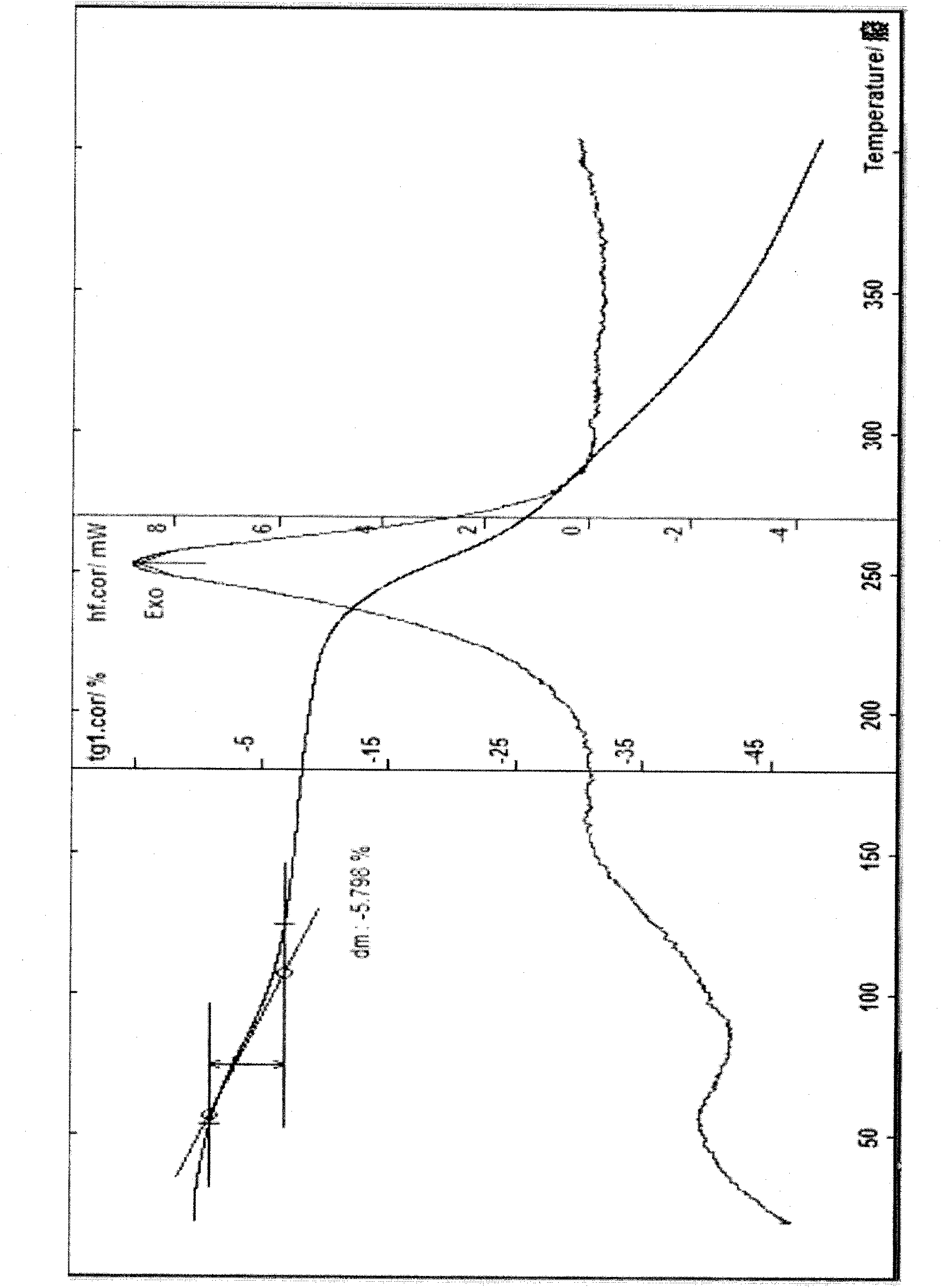
Cefodizime sodium crystal form and preparation method thereof as well as drug compound comprising crystal form
InactiveCN101759708AEasy to operateImprove liquidityAntibacterial agentsOrganic active ingredientsCefodizime SodiumDrug compound
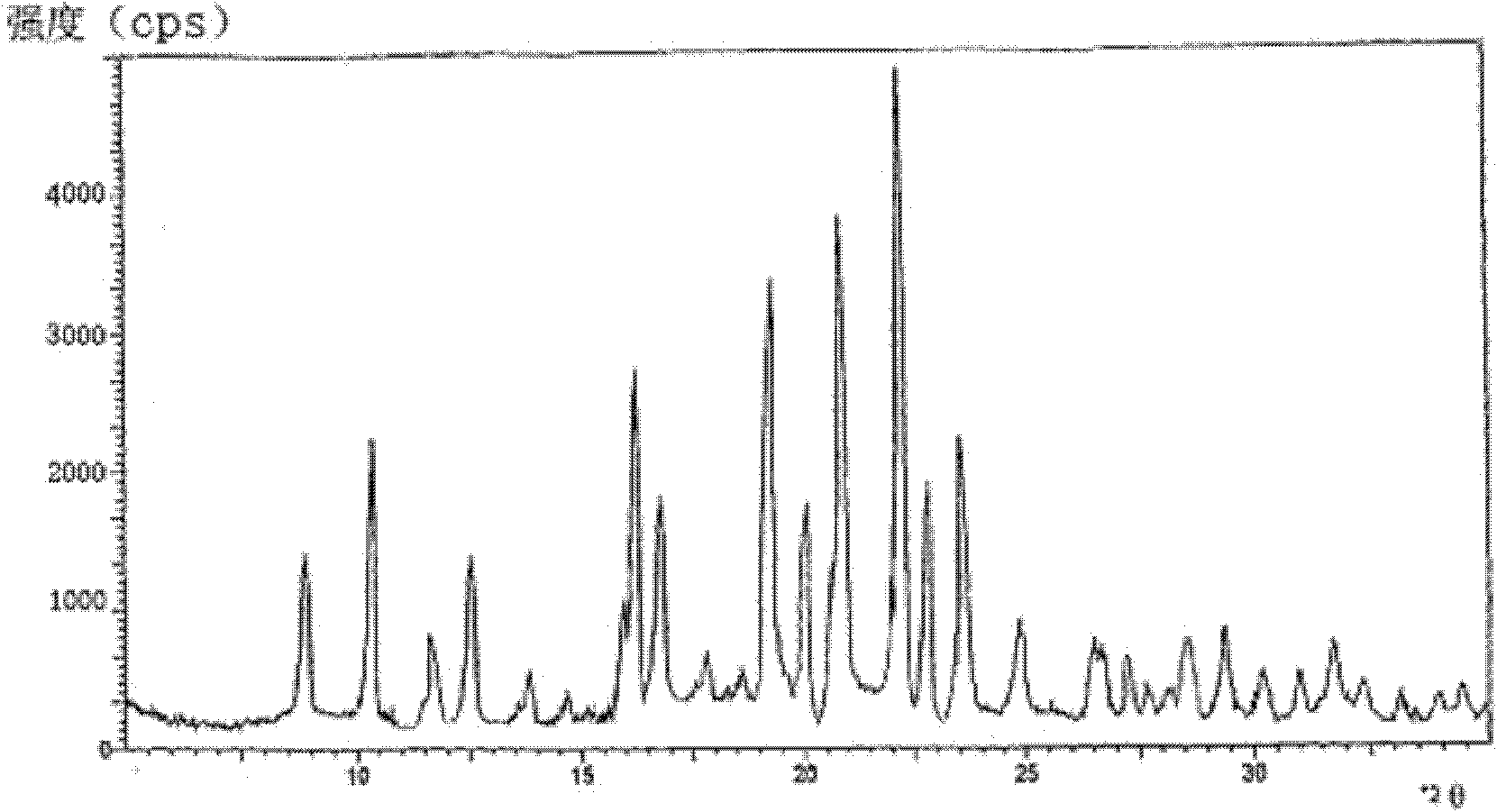
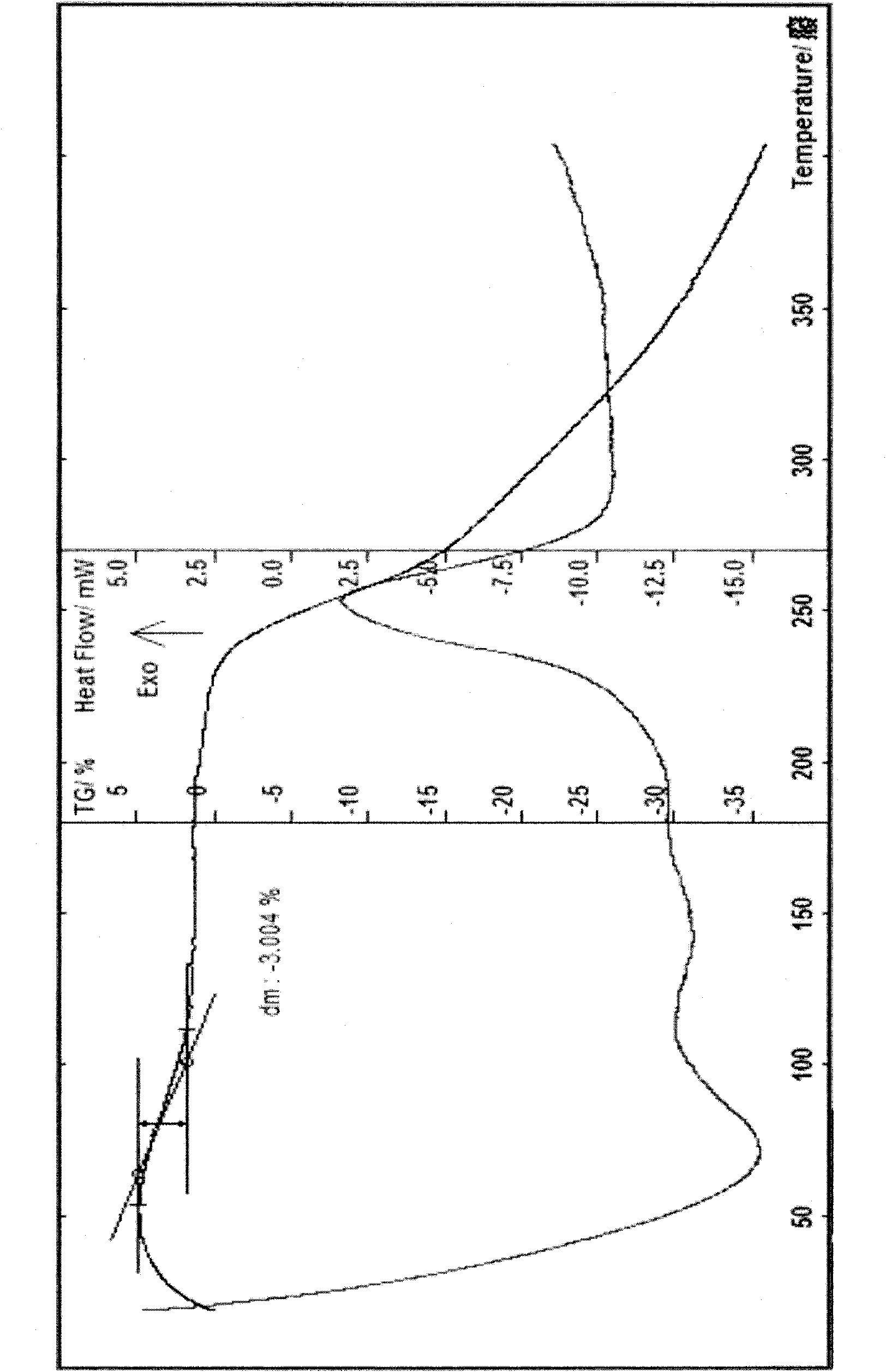
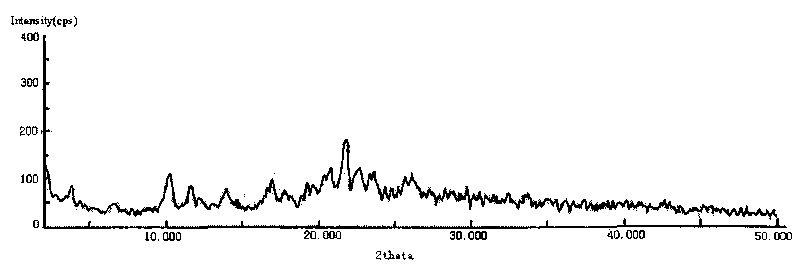
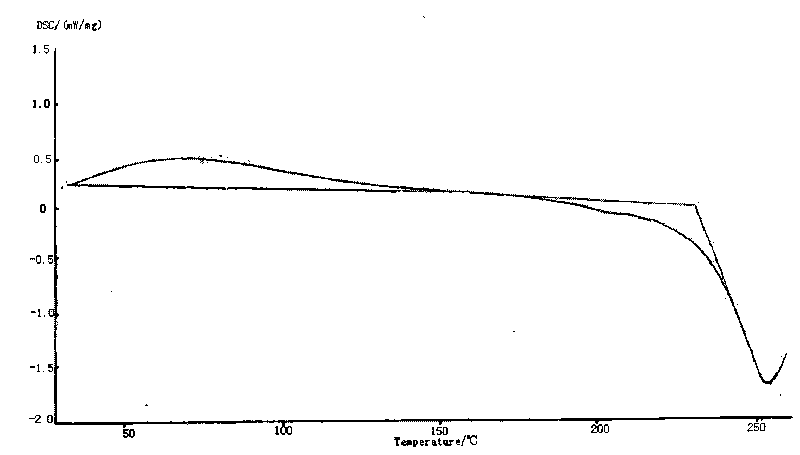
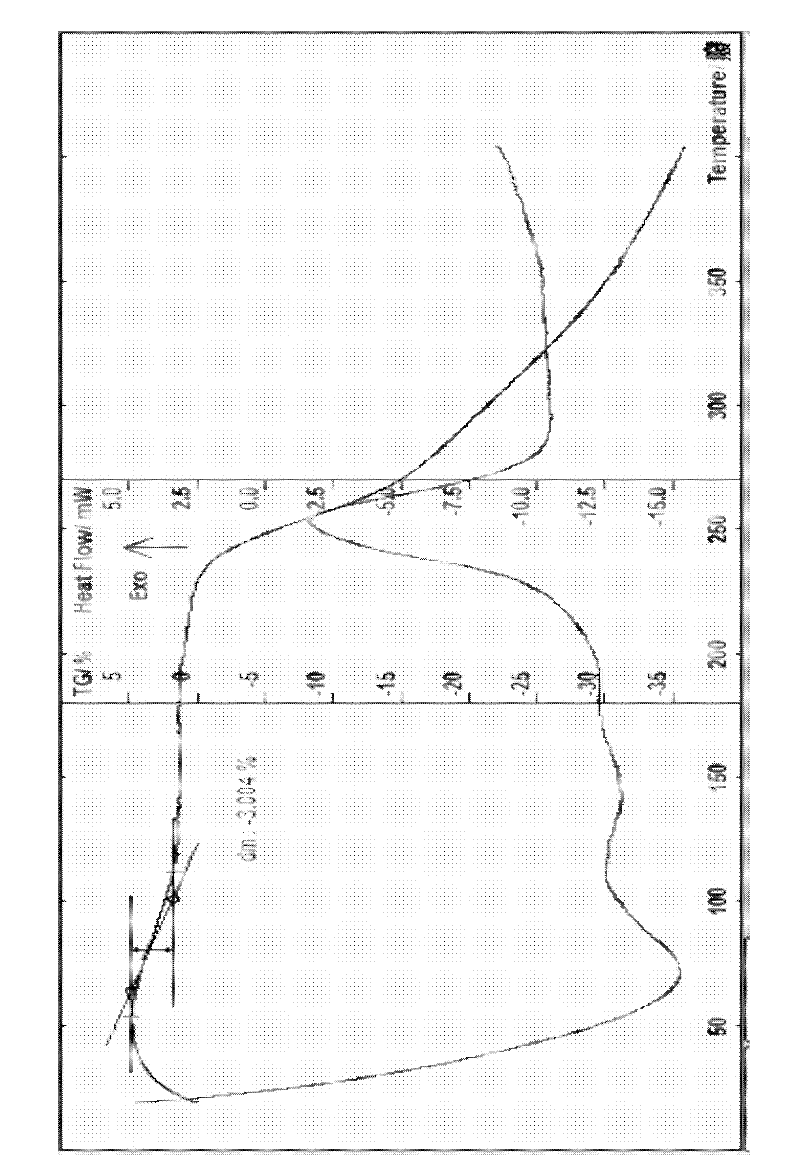
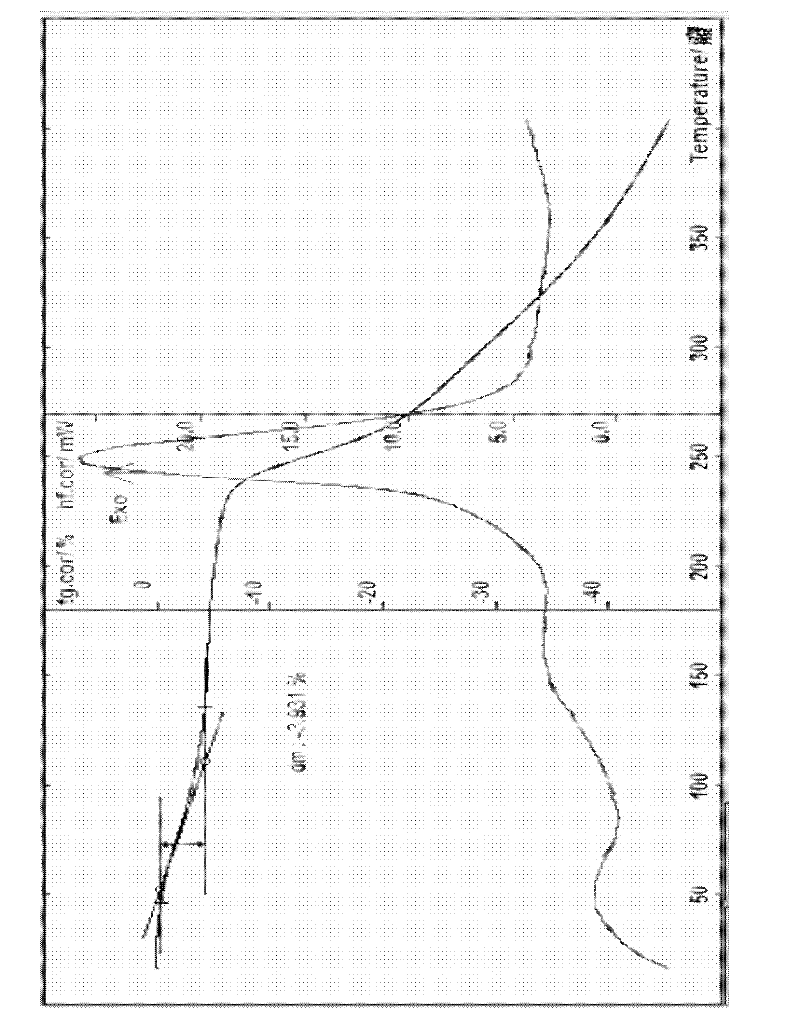
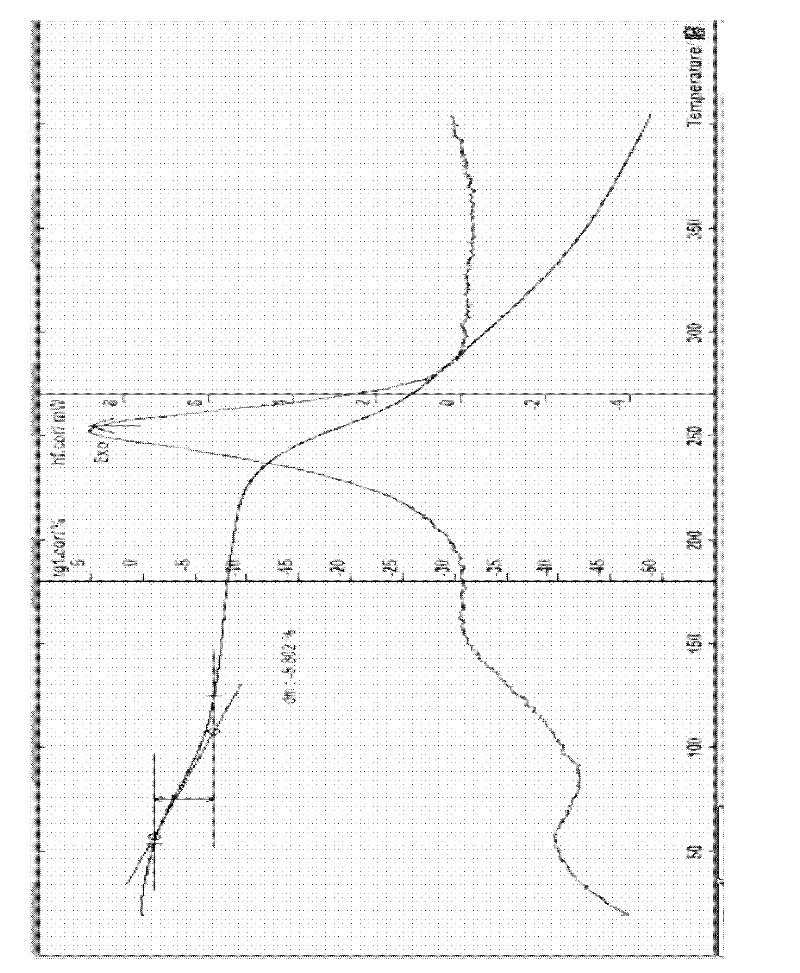
The invention provides a cefodizime sodium crystal form. In the X-ray diffraction pattern of the crystal form, a 2 theta angle is expressed to have peaks at the locations of 3.8 degrees and 6.8 degrees as well as 10.2 degrees and 21.7 degrees; the error is between plus or minus 0.2 degree; in a differential thermogram, an endothermic peak is located at 66-72 DEG C and an exothermic peak is located at 252-256 DEG C. The invention also provides a method for preparing the cefodizime sodium crystal form and a drug compound comprising the cefodizime sodium crystal form. The cefodizime sodium crystal form product of the invention is granulated and has good flowability, is not needed to be crushed when being applied to preparation, has small static electricity, and saves equipment, power and human resource costs for industrial production. The crystal form cannot be caked during drying, is easy to be operated, has low organic residue and meets relevant national standards.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Cefodizime sodium preparation method
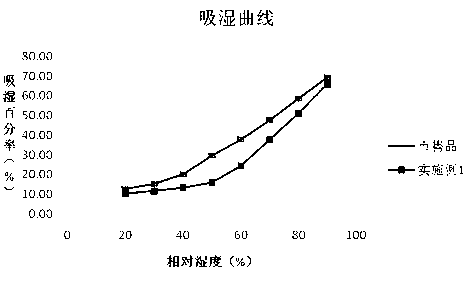
The invention belongs to the technical field of medicines, and in particular relates to a cefodizime sodium preparation method which is suitable for an industrial production of parenteral preparations. Specifically, the invention relates to a method for crystallizing and purifying the cefodizime sodium. According to the method, the purity of the cefodizime sodium is improved and the stability of the cefodizime sodium is improved by a way of crystallizing through a specific solvent and under specific conditions; more importantly, the cefodizime sodium prepared by the method is comparatively excellent in liquidity and high in critical relative humidity, and is specifically suitable for parenteral preparations of the cefodizime sodium prepared through sterile packaging; and the product has a good application prospect.
Owner:LIAONING HAISCO PHARMACEUTICAL CO LTD
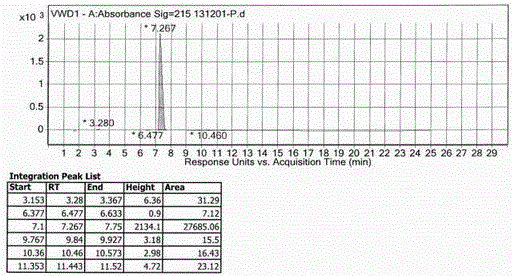
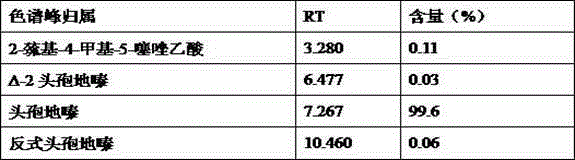
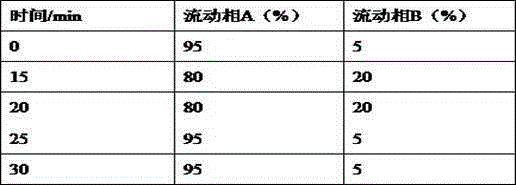
Inspection method for cefodizime sodium polymer for injection
InactiveCN104165949AFound quality problemsImprove product qualityComponent separationCefodizime SodiumGradient elution
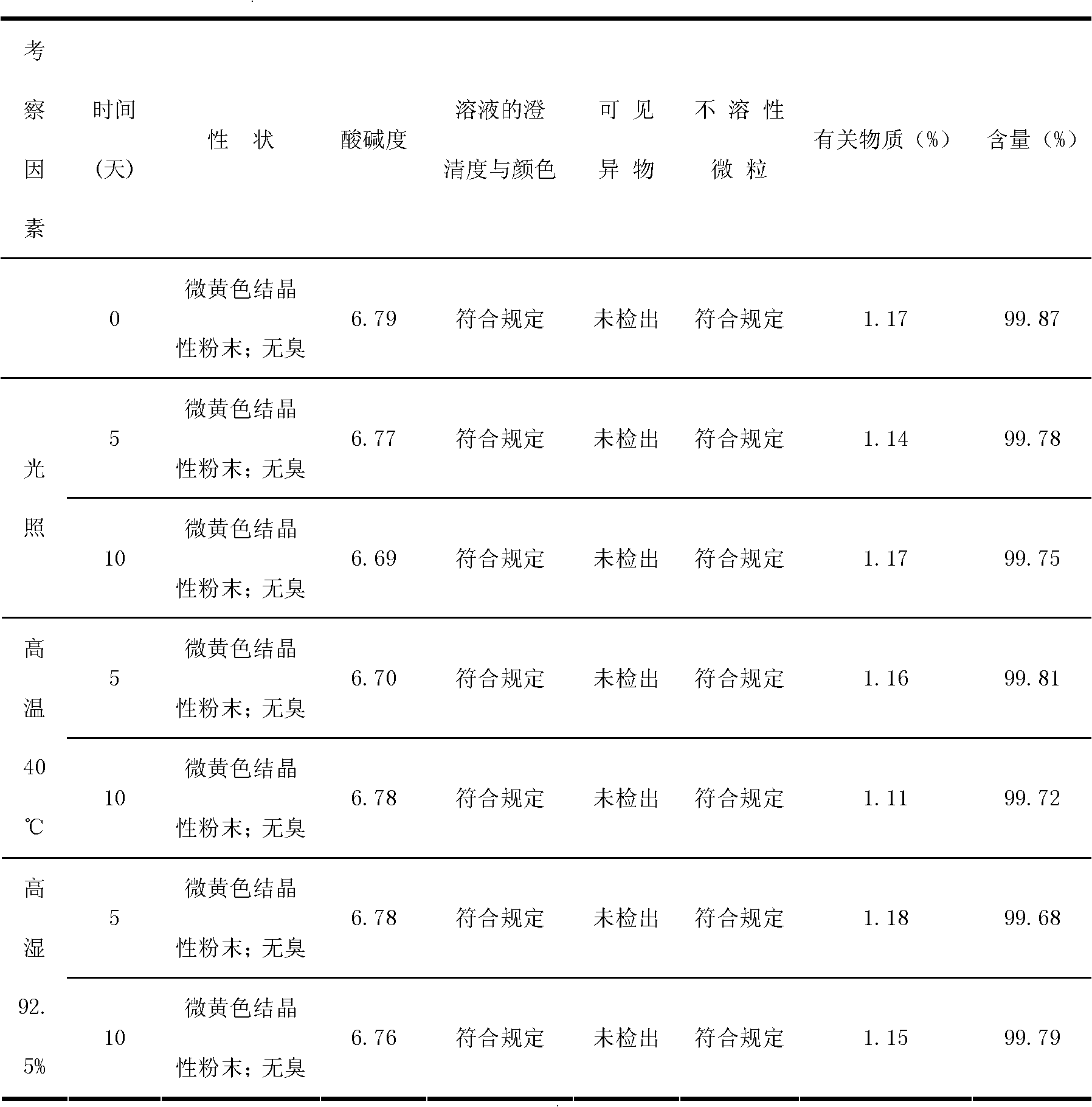
The invention discloses an inspection method for cefodizime sodium polymer for injection. The inspection method disclosed by the invention has the advantages that the amount of related substances of cefodizime sodium for injection is measured by a gradient elution method, more impurities can be detected, and the discovering of the quality problem in a pharmaceutical product is facilitated, so that the improvement of the quality of a product is improved.
Owner:SICHUAN PHARMA
Method for preparing cefodizime sodium
The invention relates to a method for preparing cefodizime sodium, and belongs to the technical field of drug synthesis. In order to solve the problems of serious environmental pollution and low yield when boron trifluoride gas or boron trifluoride complex is used as an acid catalyst, the method for preparing the cefodizime sodium includes the steps: A, reacting formula II compound 7-ACA (amino cephalosporin acid) with formula III compound MMTA under the action of concentrated sulfuric acid in acetonitrile solvents, adding sodium hydrosulfite into reaction liquid after reaction and adjusting pH (potential of hydrogen) value to range from 2.8 to 3.0 by alkaline reagents to obtain TACS; B, reacting the TACS with formula V compound AE (active ester) under the condition with organic alkali to obtain cefodizime acid; and C, performing salt forming reaction of the cefodizime acid and salt forming agents in ethanol solvents to obtain the cefodizime sodium. The method is simple in process and convenient to operate, the total yield of the cefodizime sodium reaches more than 75%, the purity of the cefodizime sodium reaches more than 99.7%, and the cefodizime sodium is fine in color and luster.
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE
Cefodizime sodium injection and preparation method thereof
InactiveCN103271880AImprove stabilityThe prescription process is simpleAntibacterial agentsPowder deliveryCefodizime SodiumFreeze-drying
The invention relates to a cefodizime sodium and preparation method thereof, especially relates to a cefodizime sodium injection for treating microbe infection, and preferably relates to a freeze-drying powder injection. The cefodizime sodium injection of the present invention is mainly composed of active component cefodizime sodium and accessories such as mannitol and citric acid. The solvent in the injection of the present invention is injection water; the excipient in the freeze-drying powder injection is mannitol, and sodium hydroxide or hydrochloric acid is used for adjusting pH valve.
Owner:张宏民
Method for controlling related substances in cefodizime sodium
InactiveCN104173291ASimple and easy quality control methodImprove product qualityAntibacterial agentsOrganic active ingredientsCefodizime SodiumQuality control
The invention provides a method for preparing cefodizime sodium for injection as well as a method for controlling related substances in the preparation method. The quality control method of the cefodizime sodium for injection disclosed by the invention is simple and feasible, stable product quality can be guaranteed, and the method is suitable for large-scale industrial production.
Owner:SICHUAN PHARMA
Cefodizime sodium for injection and preparation method of cefodizime sodium
InactiveCN110384704AThe production process is cleanImprove sanitationAntibacterial agentsPowder deliveryCefodizime SodiumPre treatment
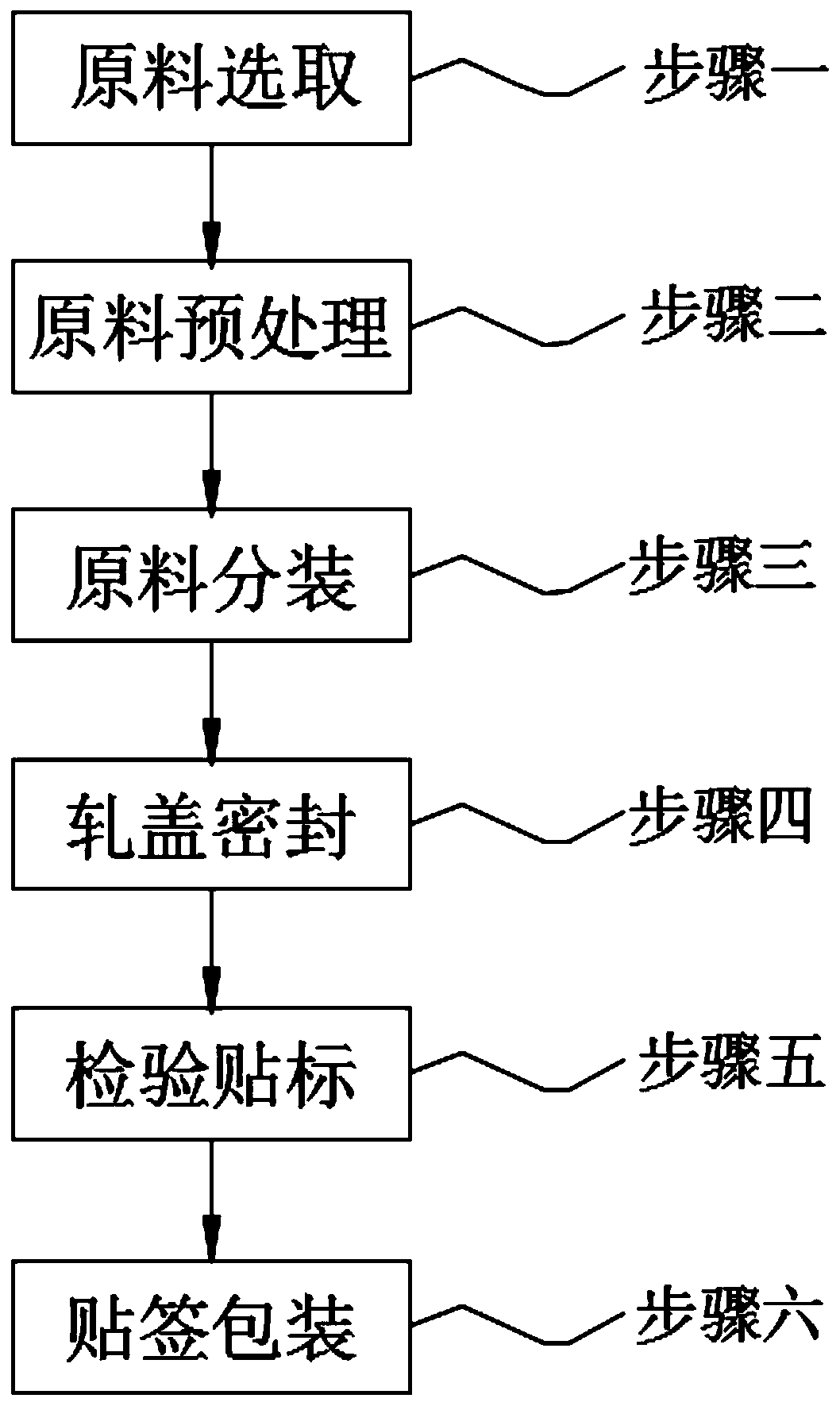
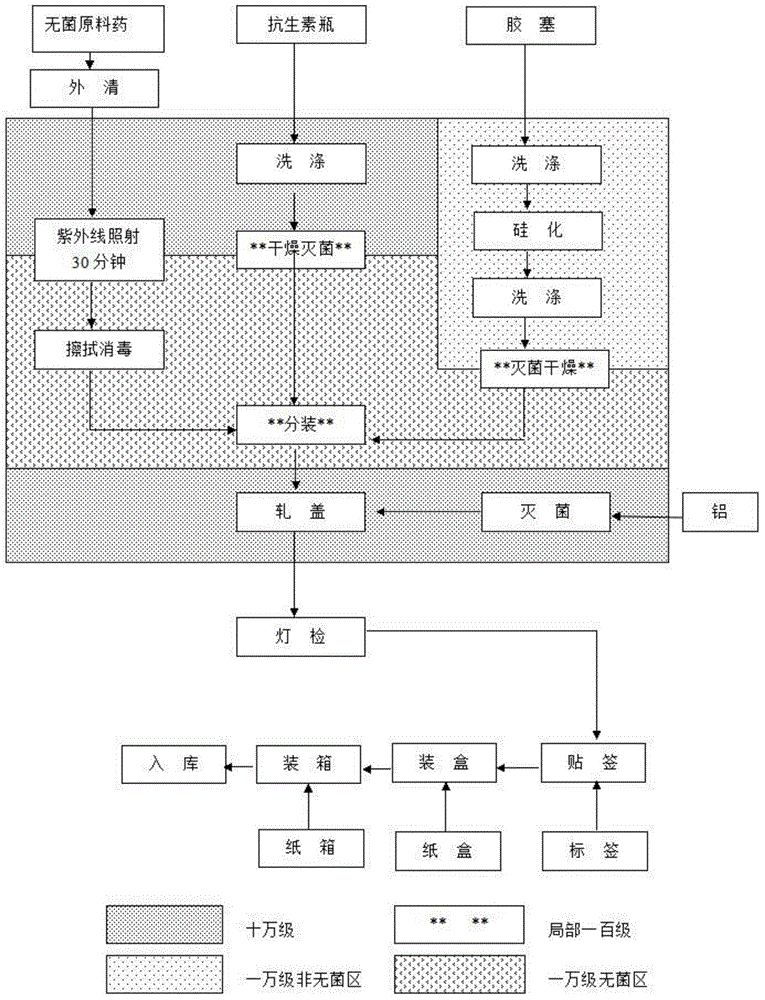
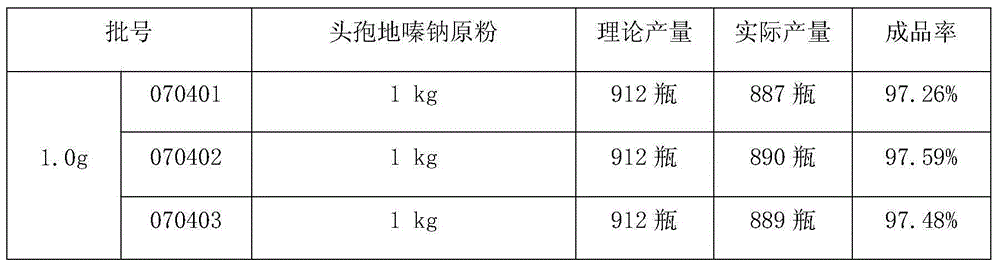
The invention discloses cefodizime sodium for injection and a preparation method of the cefodizime sodium. A dispensation comprises the cefodizime sodium, the cefodizime sodium is contained in a glassbottle, a rubber plug is plugged at the bottle opening of the glass bottle, an aluminum-plastic cover is arranged at the top of the rubber plug, and the aluminum-plastic cover is capped at the bottomopening of the glass bottle. The preparation method of the cefodizime sodium for injection comprises the following steps that 1, a raw material is selected; 2, the raw material is preprocessed; 3, the raw material is subpackaged; 4, capping and sealing are conducted; 5, detecting and marking are conducted; and 6, labelling and packing are conducted, and a raw material drug, a glass bottle, a rubber plug and a plurality of aluminum-plastic covers are selected for standby application. According to the cefodizime sodium for injection and the preparation method of the cefodizime sodium, the wholemanufacturing process is clean, the hygienic conditions are good, the manufacturing process is smooth, seamless transition is achieved, the pharmacy efficiency can be greatly improved, the process isprecise, detection is strict, the quality of products can be effectively guaranteed, meanwhile the cost is controlled, waste is less, and generalization is convenient.
Owner:汕头金石粉针剂有限公司
Pharmaceutical composition of injection cefodizime sodium and lidocaine hydrochloride injection
InactiveCN103110641AAntibacterial agentsOrganic active ingredientsCefodizime SodiumIntramuscular injection
The invention relates to a pharmaceutical composition of injection cefodizime sodium and lidocaine hydrochloride injection and in particular relates to combined application package. The pharmaceutical composition includes injection cefodizime sodium and lidocaine hydrochloride injection. During a using process, the injection cefodizime sodium is dissolved in 1% of lidocaine hydrochloride injection, so that intramuscular injection is carried out. The pharmaceutical composition not only has the broad-spectrum antibacterial action, but also can be used for relieving local pains caused by injection cefodizime sodium.
Owner:海南路易丹尼生物科技有限公司
Method for detecting cefodizime sodium related substances
ActiveCN105116076AEasy to separateGood reproducibilityComponent separationCefodizime SodiumPhosphate
The invention relates to a method for detecting cefodizime sodium related substances. Ammonium dibasic phosphate and acetonitrile are served as mobile phases to perform gradient elution; and cefodizime can be effectively separated from the related substances.
Owner:SUZHOU ERYE PHARMA CO LTD
Cefodizime sodium medicine and preparation method thereof
The invention relates to a cefodizime sodium medicine and a preparation method thereof and provides a method of preparing cefodizime. The method comprises the following steps: a, first, dissolving sodium iso-octoate in an ethanol solution, and adding a small amount of hydrochloric acid till the pH of the solution is 6.0-6.5 to obtain the ethanol solution of sodium iso-octoate; b, at normal temperature, sequentially mixing ethanol, cefodizime acid, benzophenone hydrazone, sodium metabisulfite and triethylamine, and stirring till cefodizime acid is dissolved to obtain a cefodizime acid solution; c, decoloring the obtained cefodizime acid solution, then, mixing the decolored cefodizime acid solution with the ethanol solution of sodium iso-octoate obtained in the step a, stirring, and adding a small amount of hydrochloric acid till the pH of the solution is 6.5-7.0 to obtain the ethanol solution of cefodizime sodium crystals; d, carrying out suction filtering on the obtained ethanol solution of cefodizime sodium crystals in the step c, and washing the filter cakes by anhydrous ethanol and acetone to obtain a cefodizime sodium wet material; e, drying the cefodizime sodium wet material till the water content is within 3.5%.
Owner:LIVZON PHARM GRP INC +1
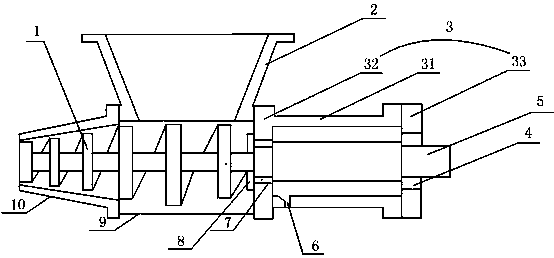
Powder feeding mechanism for preventing pollution to cefodizime sodium used for injection
The invention discloses a powder feeding mechanism for preventing pollution to cefodizime sodium used for injection. The powder feeding mechanism is provided with a powder cylinder; a transmission part is provided with a support device with an inner cavity; the support device can collect medicine powder possibly remaining in the inner cavity, so that the medicine powder is prevented from directly invading into the transmission mechanism to lead to blockage of the transmission mechanism and pollution to dry medicine powder, the transmission mechanism can be protected, and the quality of the medicine powder can be ensured.
Owner:SICHUAN PHARMA
Process for synthesizing cefodizime sodium through enzyme method
PendingCN106337075AImprove performanceLow costOrganic chemistryFermentationCefodizime SodiumAcid derivative
The invention relates to the technical field of bulk drug synthesis, particularly to a process for synthesizing cefodizime sodium through an enzyme method. According to the present invention, an immobilized cefodizime synthetase is firstly used and TACA and a 2-(2-aminothiazole-4-yl)-2-methoxyiminoacetic acid derivative are adopted as starting reactants to prepare the cefodizime sodium, such that the reaction process is complete, the reaction time is short, the yield of the reaction is increased, the quality of the cefodizime sodium is good, the purity is high, and the yield is high.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Method for preparing cefodizime sodium
ActiveCN101239985BEasy to operateLow costAntibacterial agentsOrganic active ingredientsCefodizime SodiumSolvent
The invention relates to preparation of a cefodizime sodium. The preparation includes reacting compound of formula (II) and compound of formula (III) in presence of acid catalyst to obtain compound of formula (IN); acidylating compound of formula (IN) and compound of formula (V) to obtain compound of formula (VI) in mixed solution; generating compound of formula (I) by compound of formula (VI) inpresence of salt forming agents in mixed solution. The invention is easy to operate, high in yield, and low in cost.
Owner:QILU ANTIBIOTICS PHARMA
Cefodizime sodium hydrate as well as preparation method and application thereof
The invention relates to cefodizime sodium hydrate as well as a preparation method and application thereof. The cefodizime sodium hydrate has better storage stability, and is suitable for being applied to the preparation of drugs for treating or preventing human or animal respiratory system diseases, hepatobiliary system diseases, diseases related to the five sense organs and urinary tract infection, abdominal cavity infection, pelvic cavity infection, septicemia, skin and soft tissue infections, bone and joint infections, adnexitis, intra-uterine infection, parametric connective tissue inflammation, meningitis, gonorrhea and other diseases caused by Gram-positive or negative bacteria sensitive bacteria.
Owner:胡梨芳
Determination method of related substance of cefodizime sodium for injection
InactiveCN104215714AReduce terminal absorptionReduce distractionsComponent separationCefodizime SodiumIsocratic elution
The invention discloses a determination method of related substances of cefodizime sodium for injection. With an isocratic elution method and 220nm detection length, the end adsorption of cefodizime sodium for injection is reduced, the interference of base line noise can be reduced, the accuracy and the flexibility of detection are improved, the operation is simple and the method has practical value.
Owner:SICHUAN PHARMA
Cefodizime sodium compound solid, method for preparing same and pharmaceutical preparation of cefodizime sodium compound solid
ActiveCN102796118ASimple crystallization processUniform particle size distributionAntibacterial agentsPowder deliveryActivated carbonAqueous acetone
The invention discloses a cefodizime sodium compound solid, a method for preparing the same and a pharmaceutical preparation of the cefodizime sodium compound solid, wherein the method for preparing the cefodizime sodium compound solid comprises the following steps of: first, dissolving crude cefodizime sodium salt in an acetone aqueous solution, then adding activated carbon to adsorb and filter, cooling the filtrate, stirring to separate the solid out, adding an acetone solvent to separate the solid out, filtering and drying, thus obtaining the cefodizime sodium compound solid. The prepared compound solids are fine particles, and have uniform particle size distribution and good liquidity, the crystallization technology is simple, the residual amount of organic solvent is low, and the product stability and pharmaceutical safety are effectively improved.
Owner:ZHEJIANG YATAI PHARMA
Children cefodizime sodium compound entity and preparation thereof
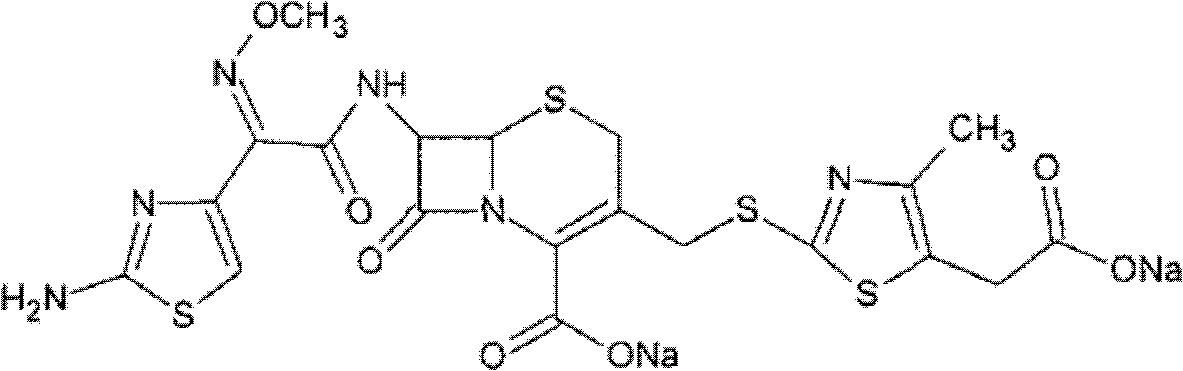
InactiveCN104961750AIncrease internal pressureImprove securityOrganic active ingredientsPowder deliveryTemperature controlCefodizime Sodium
The present invention provides a children cefodizime sodium compound entity, which has the following structural formula. The preparation method comprises: (1) dissolving a cefodizime sodium crude product in water, adding active carbon, carrying out stirring decolorization, and filtering; (2) adding an extractant under stirring, transferring and filling into a pressure resistance container, removing air bubbles, carrying out sealed oscillation, carrying out temperature control freezing, and taking out; and (3) removing the organic phase, controlling the temperature of the filtrate at 10-15 DEG C for 60 min under the protection of nitrogen gas after the solid melts, slowly adding ethanol in a dropwise manner to the filtrate, cooling to a temperature of 0-5 DEG C, growing the grain, filtering, washing, and carrying out 30 DEG C vacuum drying. The cefodizime sodium prepared through the preparation method of the present invention has characteristics of uniform particle size distribution, less impurities, and good stability.
Owner:ZHEJIANG CHANGDIAN PHARMA
Preparation method of cefodizime sodium for injection
InactiveCN104161730ASimple production processImprove product qualityAntibacterial agentsOrganic active ingredientsCefodizime SodiumChemistry
The invention provides a preparation method of cefodizime sodium for injection. Cefodizime sodium for injection provided by the invention is simple in production process, stable in quality, convenient to store and suitable for industrialized production on a large scale.
Owner:SICHUAN PHARMA
Method for refining cefodizime sodium at high yield, high cleanliness and high purity
The invention relates to a method for refining cefodizime sodium at high yield, high cleanliness and high purity. Through special design of process steps and special optimization of relevant parameters after raw material preparation and pretreatment, synthesis of cefodizime sodium, purification of cefodizime sodium and ultrahigh-cleanliness purification, the defect of difficulty in obtaining a high-purity and high-yield compound by using a general purifying and separating method is overcome, product quality is optimized, toxic and side effects are reduced, process operation is easy, high yield, high product purity and low cost are achieved, and the method is suitable for industrial production; and the purity of a product is further increased through recrystallization, so that a higher-quality product is obtained, and the safety of clinical administration is guaranteed.
Owner:TIANJIN GREENPINE PHARMA
Preparation method of cefodizime sodium
ActiveCN102816173BSolve the problem of high polymer contentHigh yieldOrganic chemistryCefodizime SodiumBiochemical engineering
The invention relates to a preparation method of cefodizime sodium. The cefodizime sodium is synthesized by using GCLE instead of traditional 7-ACA as the starting material, and the decolorized reaction solution is directly added dropwise with a salt-forming agent for reaction without separation and purification. Cefodizime sodium. The present invention has short steps and few side reactions, solves the problem of high polymer content in the final product, reduces the high polymer content from 1.0% to below 0.1%, and improves the purity. The product purity is more than 99.8%. The raw material The use of proportioning and mixed solvent improves the yield, making the product yield reach more than 90%. The invention shortens the process steps, saves time, has simple process, high yield, low cost, high product purity, cheap and easy-to-obtain raw materials, and is suitable for industrialized production.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
Cefodizime sodium proliposome preparation and preparation method thereof
InactiveCN101584664BWon't breakEncapsulation efficiency will not decreaseAntibacterial agentsOrganic active ingredientsYolkCefodizime Sodium
The invention relates to a cefodizime sodium proliposome preparation and a preparation method thereof. The proliposome preparation comprises cefodizime sodium, egg yolk lecithin, cholesterol, antioxidant and supporting agent, wherein the cefodizime sodium proliposome preparation comprises the following components by weight portion: 1 to 20 portions of the cefodizime sodium, 5 to 50 portions of theegg yolk lecithin, 3 to 30 portions of the cholesterol, 0.5 to 20 portions of the antioxidant and 3 to 50 portions of the supporting agent.
Owner:HAINAN LINGKANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com