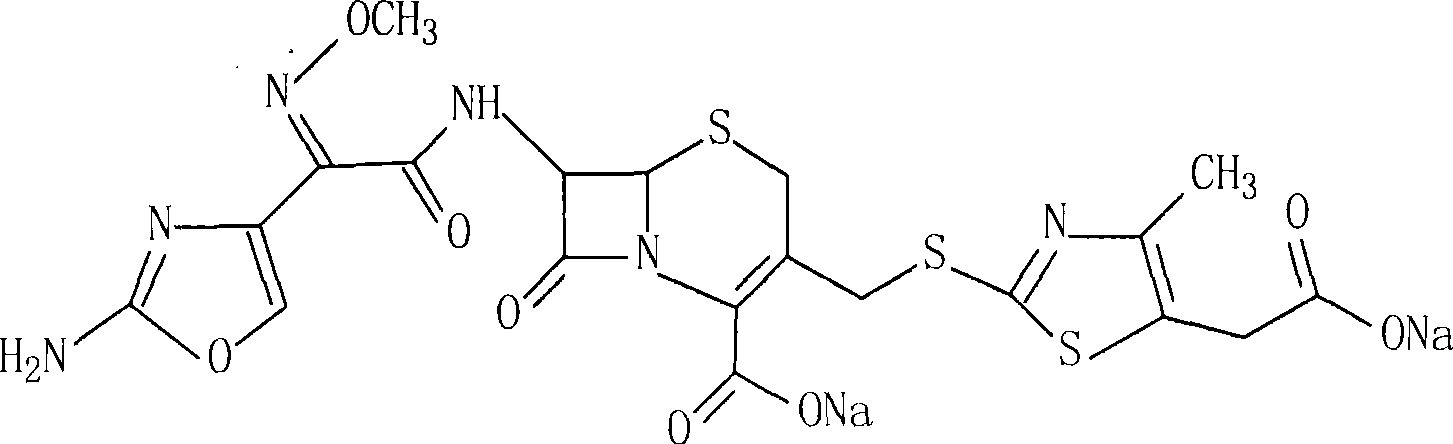
Cefodizime sodium compound and method for synthesizing the same
A technique of cefodizime sodium and a synthetic method, which is applied in the field of medicine, can solve the problems of low purity of the final product, high cost, and many operating steps, and achieve the effects of high yield, low cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1 cefodizime
[0026] 189 grams (1mol) of 2-mercapto-4-methyl-5-thiazoleacetic acid was added to 2L of an aqueous solution containing hexahydropyridine (2mol), then 455 grams (1mol) of cefotaxime was added, and stirring was continued to make the solution become Clear, heat the reaction mixture to 50-55°C, react for 4 hours, cool to room temperature, then wash twice with 500ml of ethyl acetate, separate the water phase, adjust pH=2 with 2mol / L hydrochloric acid solution, and continue stirring Solids were precipitated, filtered by suction, washed with 500 ml of water, and dried in vacuo at 45° C. to obtain 537.8 g of cefodizime with a yield of 92%.
Embodiment 2
[0027] The synthesis of embodiment 2 cefodizime sodium
[0028] Mix and stir 100 grams (0.18mol) of cefodizime acid, 200ml distilled water, and 30 grams (0.36mol) of sodium acetate. After the solution becomes clear, add 3 grams of activated carbon and continue to stir for 30 minutes, then filter to remove the activated carbon, and continue to stir the solution , while slowly adding 2L of ethanol, the solid was precipitated, filtered, washed with 300ml of ethanol, and vacuum-dried at 45°C to obtain 103.7 grams of cefodizime sodium, with a yield of 91.7% and a purity of 99.7%.
[0029] Elemental analysis theoretical value C: 38.2%, H: 2.8%, N: 13.4%, 0: 17.8%, S: 20.4%; experimental value C: 38.0%, H: 2.9%, N: 13.4%, 0: 17.9% , S: 20.3%.
[0030] MS (m / z): 627 (M-1), 606, 583, 561, 397, 378.
[0031] 1 H-NMR (DMSO-d6) δ: 9.59 (1H, d), 7.29 (2H, s) 6.75 (1H, s), 5.62 (1H, m), 5.02 (1H, d) 4.09, 4.64 (2H, m ), 3.85 (3H, s), 3.36 (4H, m), 2.18 (3H, s).
[0032] 13 C-NMR (DMSO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com