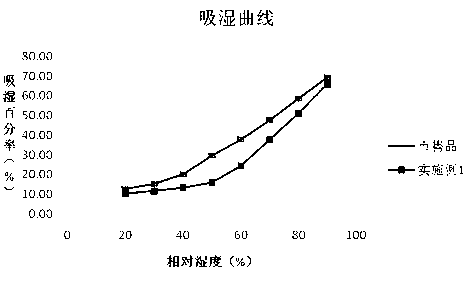
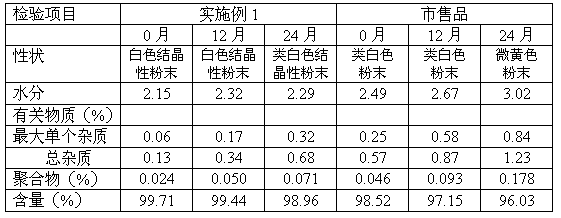
Cefodizime sodium preparation method
A technology of cefodizime sodium and n-butanol, which is applied in the field of medicine, can solve the problems of unfavorable mechanical sub-packaging and loading control, increased equipment and power costs, and low critical relative humidity, achieving good application prospects, improving stability, The effect of reducing production cost and difficulty of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Add the crude product of cefodizime sodium (commercially available cefodizime sodium) into the mixed solution of water and n-butanol, wherein the weight ratio of the crude product of cefodizime sodium, water and n-butanol is 1:1.5:3. Heat and stir at 45-50°C until dissolved to obtain a water-n-butanol solution of cefodizime sodium;
[0025] (2) Decolorization treatment: adding activated carbon in an amount of 0.3% of the total volume of the solution to the water-n-butanol solution of cefodizime sodium for decolorization treatment, and then filtering out insoluble impurities while hot to obtain a decolorization solution;
[0026] (3) Cool the decolorizing solution to 20-25°C, crystallize at a constant temperature of 20-25°C, filter, and vacuum-dry at 40°C for about 5 hours to obtain the finished product.
Embodiment 2
[0028] (1) Add the crude cefodizime sodium to the mixed solution of water and n-butanol, wherein the weight ratio of the crude cefodizime sodium, water and n-butanol is 1:1.5:3, heat and stir at 45-50°C until dissolved , to obtain the water-n-butanol solution of cefodizime sodium;
[0029] (2) Decolorization treatment: add activated carbon in an amount of 0.5% of the total volume of the solution to the water-n-butanol solution of cefodizime sodium for decolorization treatment, and then filter out insoluble impurities while hot to obtain a decolorization solution;
[0030] (3) Cool the decolorizing solution to 20-25°C, crystallize at a constant temperature of 20-25°C, filter, and vacuum-dry at 40°C for about 5 hours to obtain the finished product.
Embodiment 3
[0032] (1) Add the crude cefodizime sodium to the mixed solution of water and n-butanol, wherein the weight ratio of the crude cefodizime sodium, water and n-butanol is 1:1.5:3, heat and stir at 45-50°C until dissolved , to obtain the water-n-butanol solution of cefodizime sodium;
[0033] (2) Decolorization treatment: add activated carbon in an amount of 0.1% of the total volume of the solution to the water-n-butanol solution of cefodizime sodium for decolorization treatment, and then filter out insoluble impurities while hot to obtain a decolorization solution;
[0034] (3) Cool the decolorizing solution to 20-25°C, crystallize at a constant temperature of 20-25°C, filter, and vacuum-dry at 40°C for about 5 hours to obtain the finished product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluidity | aaaaa | aaaaa |
| fluidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com