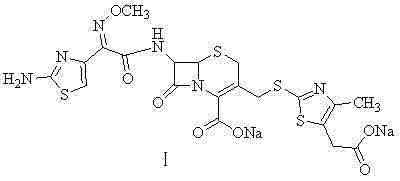
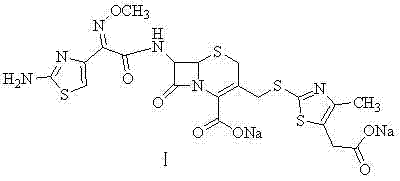
Preparation method of cefodizime sodium
A technology for cefodizime and cefodizime acid, applied in the field of drug synthesis, can solve the problems of many operation steps, low recovery rate, high price and the like, and achieve the effects of ensuring product quality and yield, improving yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
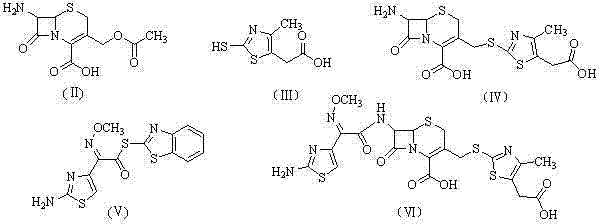
[0042] Synthesis of 7-amino-3-(5-carboxymethyl-4-methyl-1,3-thiazole-2-mercaptomethyl)cephalosporin-2-ene-2carboxylic acid (Ⅳ)
[0043] In a 1000ml three-neck reaction flask, add 300ml of distilled water, 7-ACA (54.4g, 0.2mol) and sodium bicarbonate (16.8g, 0.2mol), and add 2-mercapto-4-methyl-5-thiazoleacetic acid ( 41.4g, 0.2mol), sodium bicarbonate (16.8g, 0.2mol) distilled water 200ml, adjust the pH to 6.5 with saturated sodium bicarbonate solution and keep it until the end of the reaction, react at 40-45°C for 2h, cool to room temperature, add 30ml For n-butanol, adjust the pH to 5.5 with 15% dilute sulfuric acid, stir for 20 minutes, further adjust the pH to 4, stir for 1 hour, filter with suction, wash with distilled water, isopropanol, and n-hexane in turn, and dry to obtain 65.0 g of off-white crystalline powder. Yield 80.9%.
Embodiment 2
[0045] Synthesis of Cefodizime Acid (Ⅵ)
[0046] Add 300ml of dichloromethane into a 500ml reaction flask, add intermediate (IV) (40.1g, 0.1mol), 28ml of triethylamine and AE active ester (42.0g, 0.12mol) while stirring at room temperature, and react at the same temperature for 2h. Add water 200ml×3 for extraction, combine the water layers, decolorize with activated carbon, filter, adjust the pH of the filtrate to 3.5 with hydrochloric acid, stir for 1.5h, filter with suction, wash with 250ml of water and 100ml of isopropanol, and dry to obtain an off-white solid 53.0 g, yield 90.6%.
Embodiment 3
[0048] Synthesis of Cefodizime Sodium (Ⅰ)
[0049] At 8-10°C, add 35.04g of cefodizime acid and 18ml of triethylamine to 250ml of ethanol in turn, add dropwise 15ml of water, then add 1g of activated carbon and stir for 20min, filter with suction, and dissolve 20.3g of sodium isooctanoate in In 90ml of ethanol, add the above-mentioned sodium isooctanoate solution dropwise to the obtained filtrate at 10-20°C for 1 hour. After the dropwise addition is completed, continue stirring at room temperature for 1.5 hours, filter with suction, wash with 200ml of ethanol, and dry in vacuo at 45°C , to obtain cefodizime sodium 32.3g, yield 85.0%.
[0050] The structure was confirmed by NMR and mass spectrometry
[0051] 1 H-NMR (DMSO-d 6 )δ: 9.59(1H, d), 7.29(2H, s), 6.74(1H, s), 5.62(1H, m), 5.02(1H, d), 4.09, 4.63(2H, m), 3.85(3H , s), 3.35(4H, m), 2.18(3H, s).
[0052] 13 C-NMR (DMSO-d 6 ) δ: 172.5, 168.0, 164.5, 162.5, 159.3, 148.5, 145.6, 142.0, 132.5, 128.8, 115.9, 108.6, 61.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com