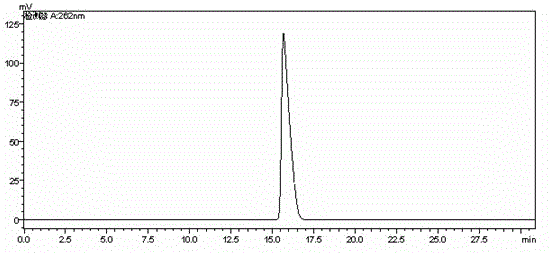
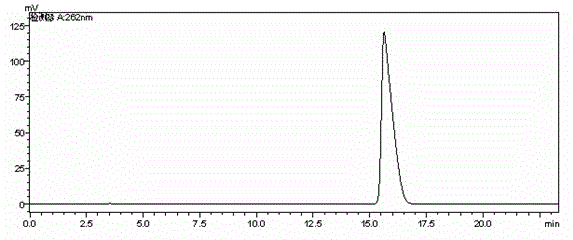
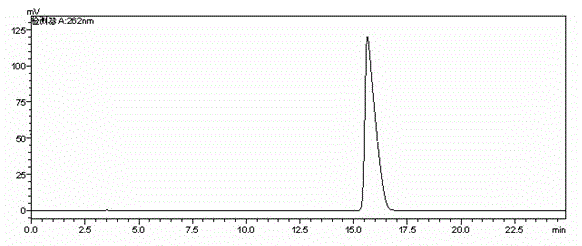
Preparation method of cefodizime sodium
A technology for cefodizime sodium and a compound is applied in the field of chemical drug synthesis, which can solve the problems of short synthesis reaction steps and high polymer content, and achieve the effects of low product cost, high product yield and time saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of cefodizime sodium adopts compound I as a starting material, and the specific steps include:
[0035] (1) Add 30g of compound I (GCLE) and 12.3g of compound II into a system containing 200mL of acetone and 20mL of water, heat to 50°C, stir until clear, add 0.3g of sodium iodide, and use a mass fraction of 10% sodium carbonate Adjust the pH of the solution to 5.0, react for 2 hours, add 100 g of trifluoroacetic acid for hydrolysis, add 1.5 g of activated carbon for decolorization, wash with a mixture of 10 mL of acetone and 1 mL of water, adjust the pH to 3.0 with 25% ammonia water, and culture at 10 °C. crystallize for 1 hour, filter with suction, wash with 100mL of water and 100mL of acetone respectively, and dry in vacuo to obtain 22.1g of compound III with a yield of 92% and a purity of 98.76%;
[0036] (2) Add 20g of compound III and 27.4g of compound IV into a mixed solvent system containing 100mL of toluene and 100mL of methanol, dropwise ...
Embodiment 2
[0039] The preparation method of cefodizime sodium adopts compound I as a starting material, and the specific steps include:
[0040] (1) Add 30g of compound I (GCLE) and 13.2g of compound II into a system containing 200mL of acetone and 15mL of water, heat to 50°C, stir until clear, add 0.7g of sodium bromide, and use a mass fraction of 8% bicarbonate Adjust the pH to 5.5 with sodium solution, react for 1 hour, add 141g of trifluoroacetic acid for hydrolysis, add 1.5g of activated carbon for decolorization, wash with 10mL of acetone and 0.75mL of water mixture, adjust the pH to 3.5 with 28% ammonia water, 12°C Cultivate the crystal for 1 hour, filter with suction, wash with 100 mL of water and 100 mL of acetone respectively, and dry in vacuo to obtain 21.8 g of compound III with a yield of 91% and a purity of 98.62%;
[0041] (2) Add 20g of compound III and 34.2g of compound IV into a mixed solvent system containing 200mL of toluene and 100mL of ethanol, add dropwise 23mL of ...
Embodiment 3
[0044] The preparation method of cefodizime sodium adopts compound I as a starting material, and the specific steps include:
[0045] (1) Add 30g of compound I (GCLE) and 14.1g of compound II into a system containing 200mL of acetone and 10mL of water, heat to 50°C, stir until clear, add 0.9g of potassium bromide, and use 12% carbonic acid Adjust the pH to 5.2 with sodium hydrogen solution, react for 2 hours, add 54 g of trifluoroacetic acid for hydrolysis, add 1.5 g of activated carbon for decolorization, wash with a mixture of 10 mL of acetone and 1 mL of water, adjust the pH to 3.5 with 26% ammonia water, and heat at 15 °C Cultivate the crystal for 1 hour, filter with suction, wash with 100 mL of water and 100 mL of acetone respectively, and dry in vacuo to obtain 21.5 g of compound III with a yield of 90% and a purity of 98.56%;
[0046] (2) Add 20g of compound III and 30g of compound IV into a mixed solvent system containing 100mL of acetone and 100mL of methanol, dropwis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com