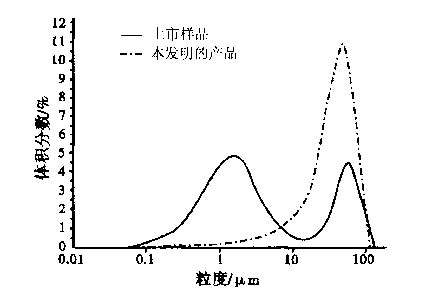
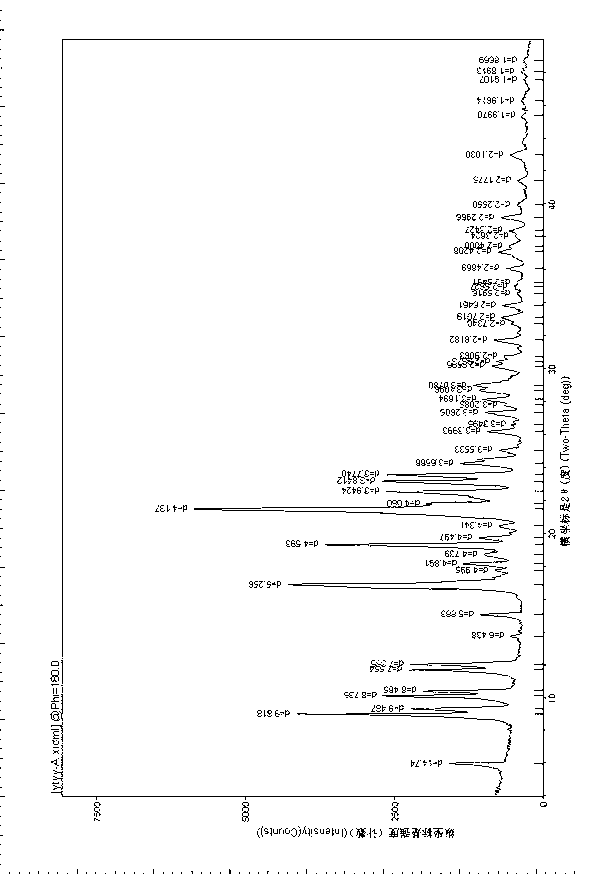
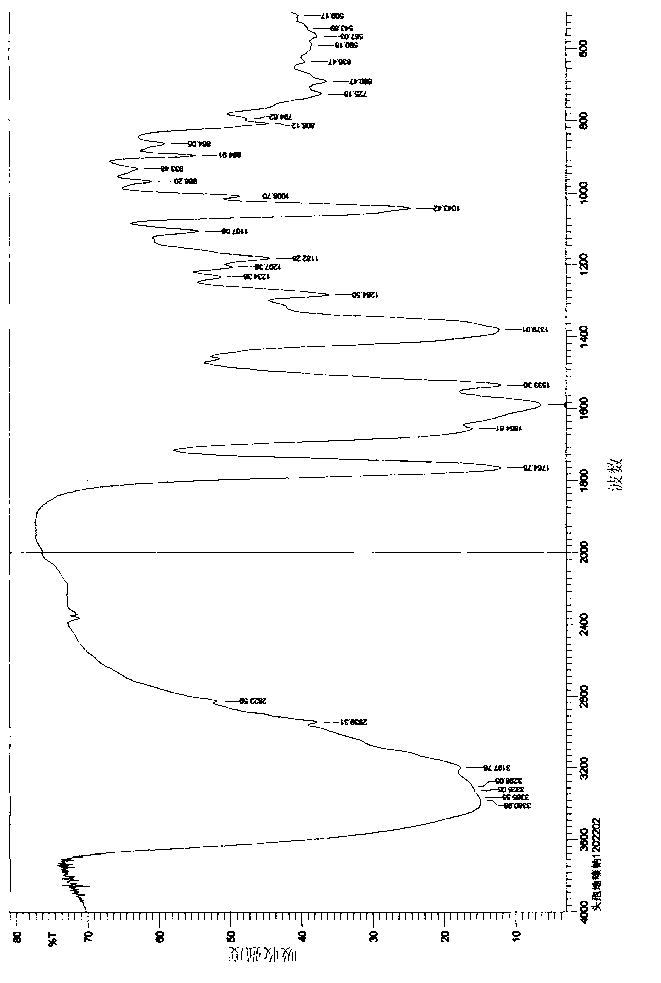
Cefodizime sodium compound solid, method for preparing same and pharmaceutical preparation of cefodizime sodium compound solid
A technology of cefodizime sodium and pharmaceutical preparations, which is applied in the fields of cefodizime sodium compound entities, cefodizime sodium crystal forms, preparation methods and pharmaceutical preparations, and can solve the problems of unstable product loading, increased vascular irritation, and residual To solve the problem of high quantity, achieve the effect of improving product stability and drug safety, improving drug safety, and simplifying the crystallization process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation method of a cefodizime sodium compound entity of the present embodiment comprises the steps of: taking 10 g of crude cefodizime sodium salt, adding 60 ml of acetone aqueous solution with a volume fraction of 35%, and stirring at room temperature until completely dissolved; then adding 1.0 g Activated carbon is used for adsorption and filtration, and the filtered solution is placed in an ice bath to cool down to 10°C, and stirred for 20 minutes until a large amount of crystals are precipitated; slowly add 300ml of acetone solvent with a mass concentration of 95% to the above crystal precipitation container within 50 minutes , to make it all dissolve, place the solution in an ice bath, cool down to about 0°C, stir at a stirring speed of 100rpm until the solids are completely precipitated, and vacuum-dry to constant weight through suction filtration, wherein the drying adopts vacuum drying at a temperature of 43°C , -0.05MPa, dried for 4h; 7.8g fine granular...
Embodiment 2
[0029] The preparation method of a cefodizime sodium compound entity of the present embodiment comprises the following steps: take 10 g of crude cefodizime sodium salt, add 65 ml of acetone aqueous solution with a volume fraction of 40%, stir at room temperature until completely dissolved; then add 1.0 g Activated carbon is used for adsorption and filtration, and the filtered solution is placed in an ice bath to cool down to 10°C, and stirred for 20 minutes until a large amount of crystals are precipitated; slowly add 300ml of acetone solvent with a mass concentration of 95% to the above crystal precipitation container within 50 minutes , to make it all dissolve, put the solution in an ice bath, cool down to 5°C, stir at a stirring speed of 90rpm until the solid is completely precipitated, and vacuum-dry to constant weight through suction filtration, wherein the drying is vacuum drying, the temperature is 45°C, -0.05MPa, dried for 3 hours; 7.4g fine granular cefodizime sodium c...
Embodiment 3
[0032] The preparation method of a kind of cefodizime sodium compound entity of the present embodiment comprises the following steps: take 10 g of crude cefodizime sodium salt, add 50 ml of acetone aqueous solution with a volume fraction of 50%, stir at room temperature until completely dissolved; then add 1.0 g Adsorb and filter the activated carbon, put the filtrate in an ice bath and cool down to 8°C, stir for 20 minutes until a large amount of crystals are precipitated; slowly add 300ml of acetone solvent with a mass concentration of 95% to the crystal precipitation container within 50 minutes , to make it all dissolve, place the solution in an ice bath, cool down to -5°C, stir at a stirring speed of 160rpm until the solids are completely precipitated, and vacuum-dry to constant weight through suction filtration, wherein the drying is vacuum drying at 40°C , -0.05MPa, dried for 5h; 7.0g fine granular cefodizime sodium compound solid product was obtained. The X-ray diffract...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com