Tedizolid ammonium phosphate and its crystal form, preparation method and medical application
A technology of tedizolid ammonium phosphate and crystal form, which is applied in the field of chemical pharmaceuticals, and can solve problems such as unfavorable filtration and drying, unsatisfactory removal of impurities, and small particle size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
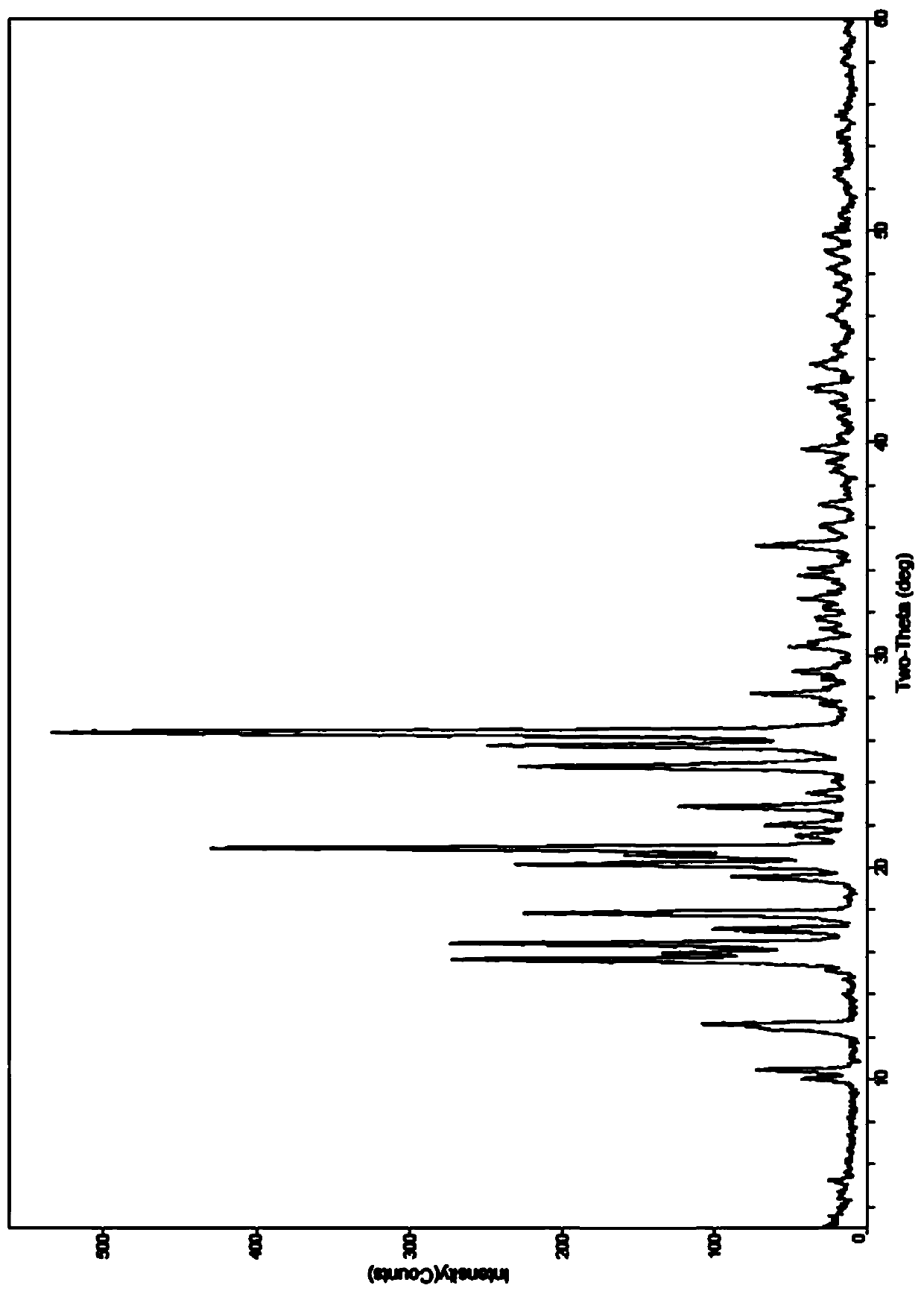
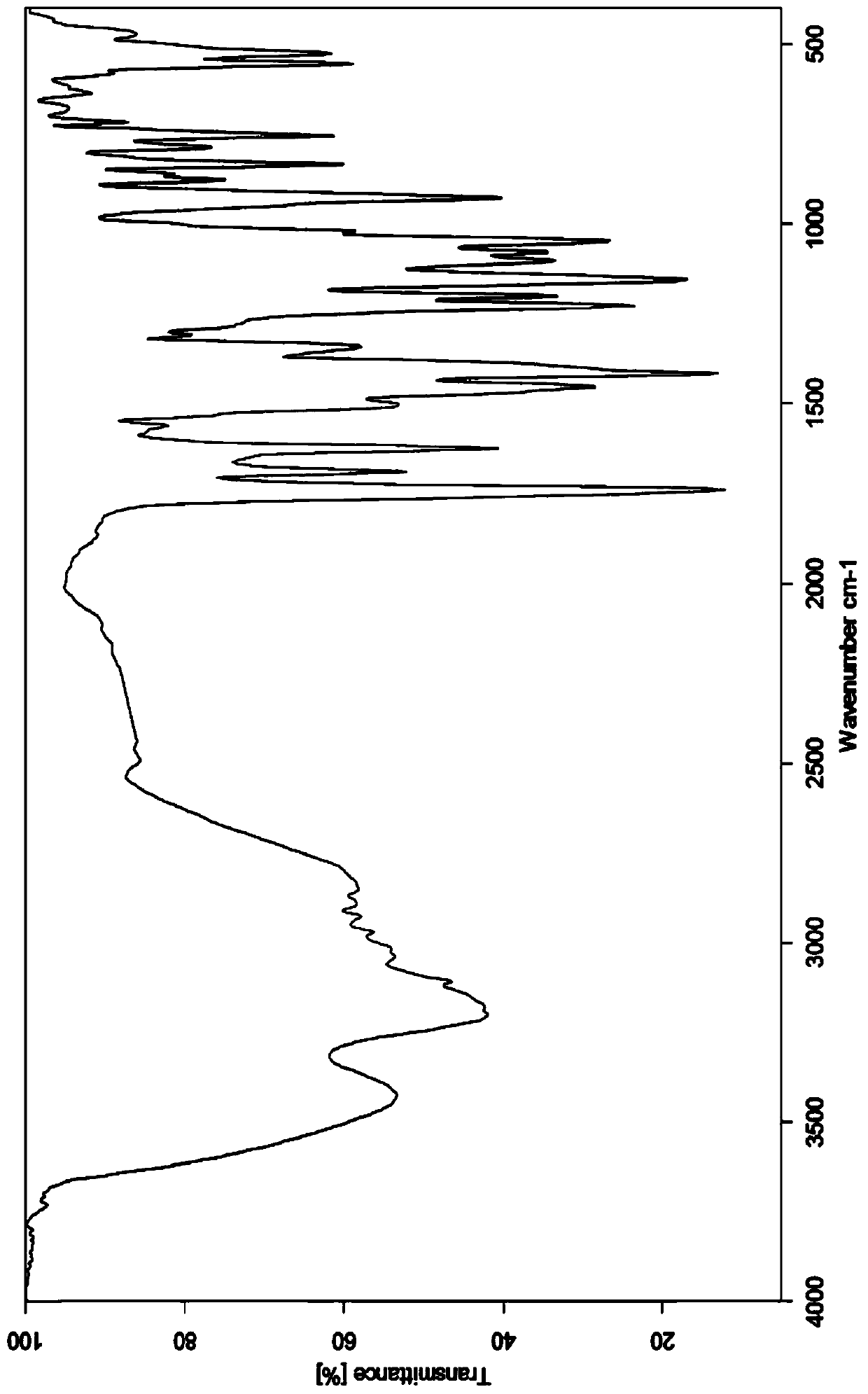
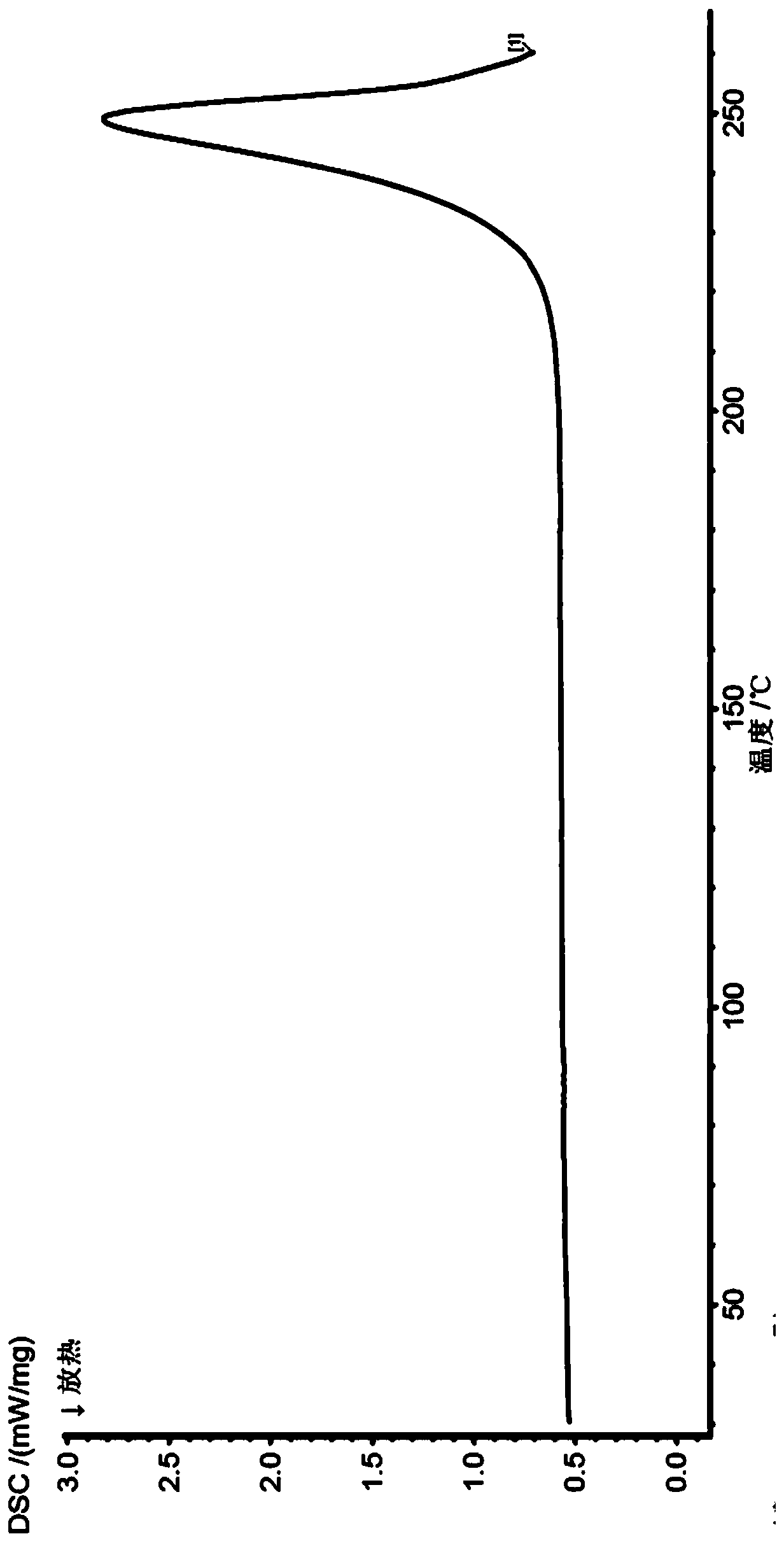
Image
Examples
Embodiment 1
[0068] Dissolve 5 g of tedizolid phosphate crude product (HPLC purity>98%) in 22 ml of 2% ammonia solution at room temperature, and the molar ratio of tedizolid phosphate and ammonia water in the ammonia solution is 1:2 to obtain tedizolid phosphate The crude product of tedizolid ammonium salt was 3.8g, and the obtained tedizolid ammonium phosphate was easy to filter, not agglomerated, and easy to dry.
[0069] ESI-MS 448.89[M-H] - ;
[0070] 1 HNMR (DMSO, 400MHz) δ: 8.92(s, 1H), 8.20(m, 2H), 7.73(m, 2H), 7.52(d, 1H), 7.26(br s, 5H), 4.90(m, 1H) , 4.48(s, 3H), 4.17(t, 1H), 3.99(t, 1H), 3.94(m, 2H).
Embodiment 2
[0072] Dissolve 5 g of tedizolid phosphate crude product (HPLC purity>98%) in 220 ml of 0.1% ammonia solution at room temperature, and the molar ratio of tedizolid phosphate and ammonia water in the ammonia solution is 1:1 to obtain tedizolid phosphate The crude product of tedizolid ammonium salt is 3.2 g, and the obtained tedizolid ammonium phosphate is easy to filter, does not agglomerate, and is easy to dry.
[0073] ESI-MS 448.89[M-H] - ;
[0074] 1 HNMR (DMSO, 400MHz) δ: 8.92(s, 1H), 8.20(m, 2H), 7.73(m, 2H), 7.52(d, 1H), 7.26(br s, 5H), 4.90(m, 1H) , 4.48(s, 3H), 4.17(t, 1H), 3.99(t, 1H), 3.94(m, 2H).
Embodiment 3
[0076]5 g of tedizolid phosphate crude product (HPLC purity>98%) was dissolved in 11 ml of 18% ammonia solution at room temperature, and the molar ratio of tedizolid phosphate and ammonia water in the ammonia solution was 1:9 to obtain tedizolid phosphate The crude product of tedizolid ammonium salt was 4.3g, and the obtained tedizolid ammonium phosphate was easy to filter, not agglomerated, and easy to dry.
[0077] ESI-MS 448.89[M-H] - ;
[0078] 1 HNMR (DMSO, 400MHz) δ: 8.92(s, 1H), 8.20(m, 2H), 7.73(m, 2H), 7.52(d, 1H), 7.26(br s, 5H), 4.90(m, 1H) , 4.48(s, 3H), 4.17(t, 1H), 3.99(t, 1H), 3.94(m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com