Patents
Literature
40results about How to "Simple crystallization process" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
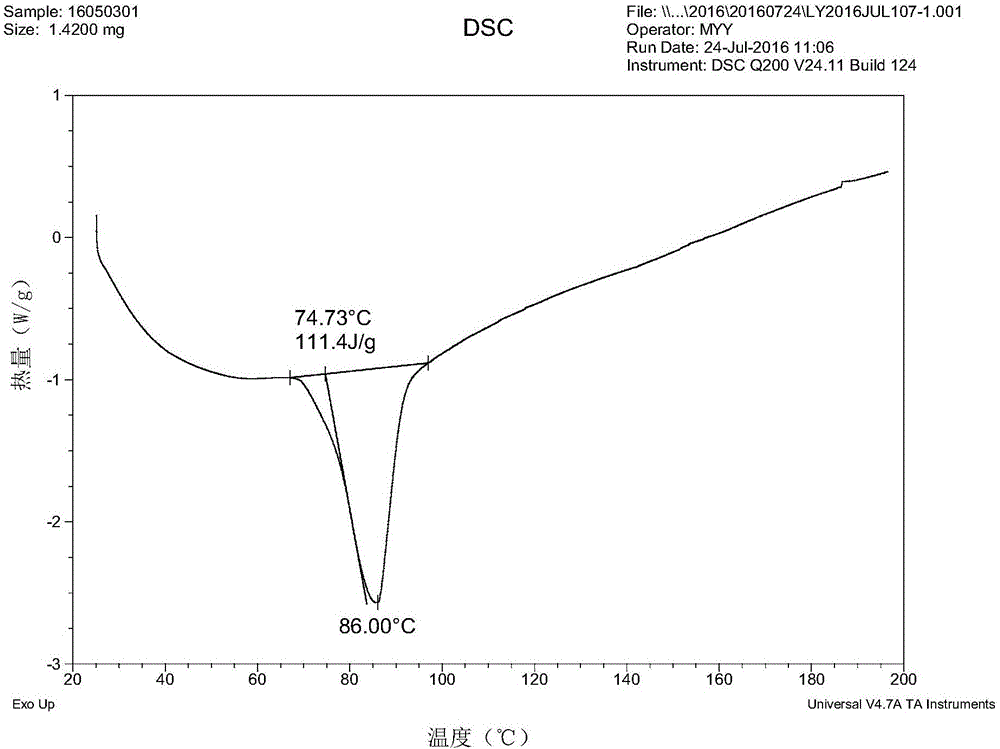
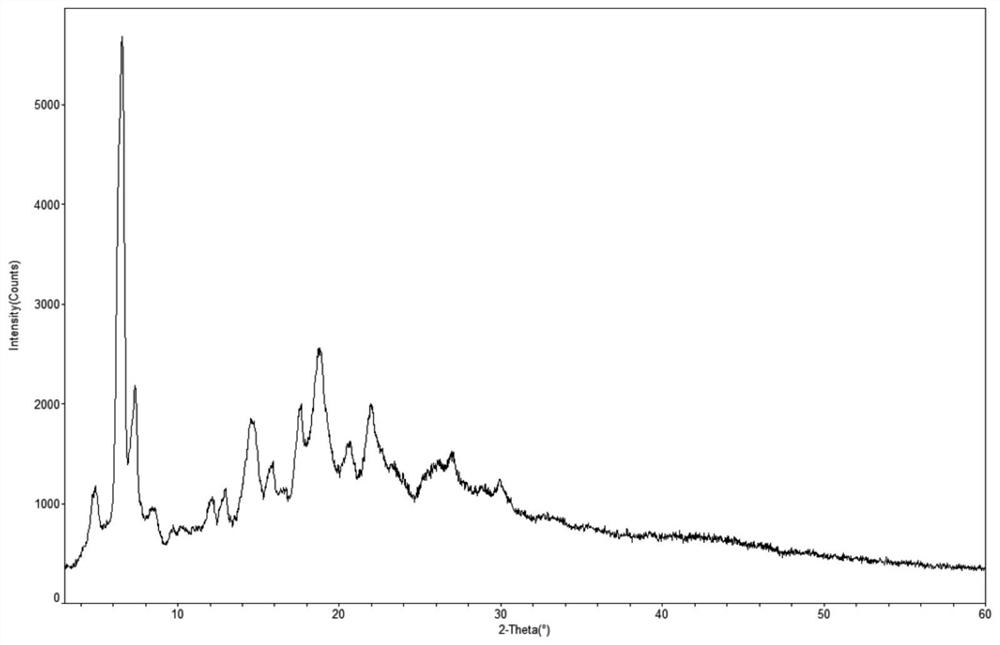
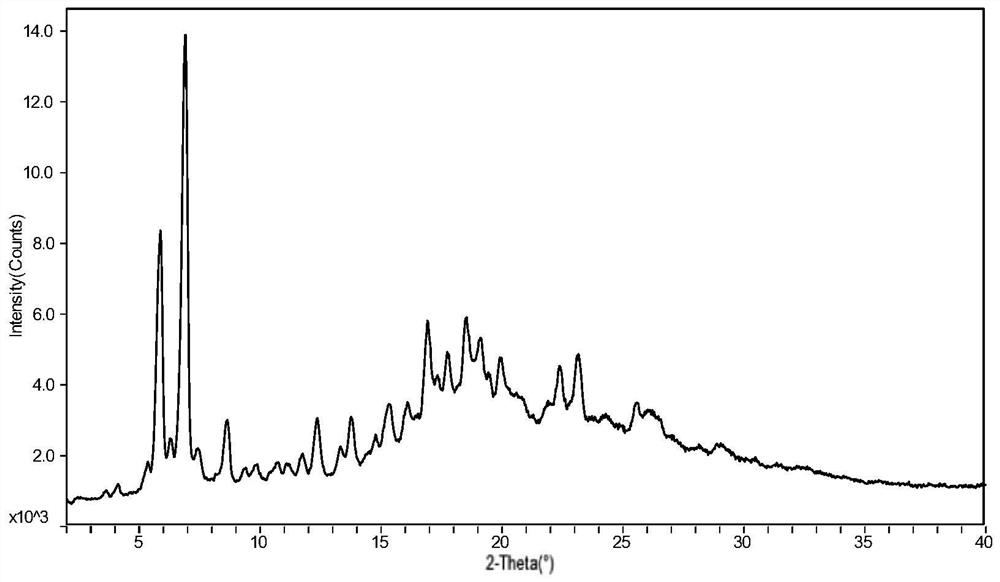
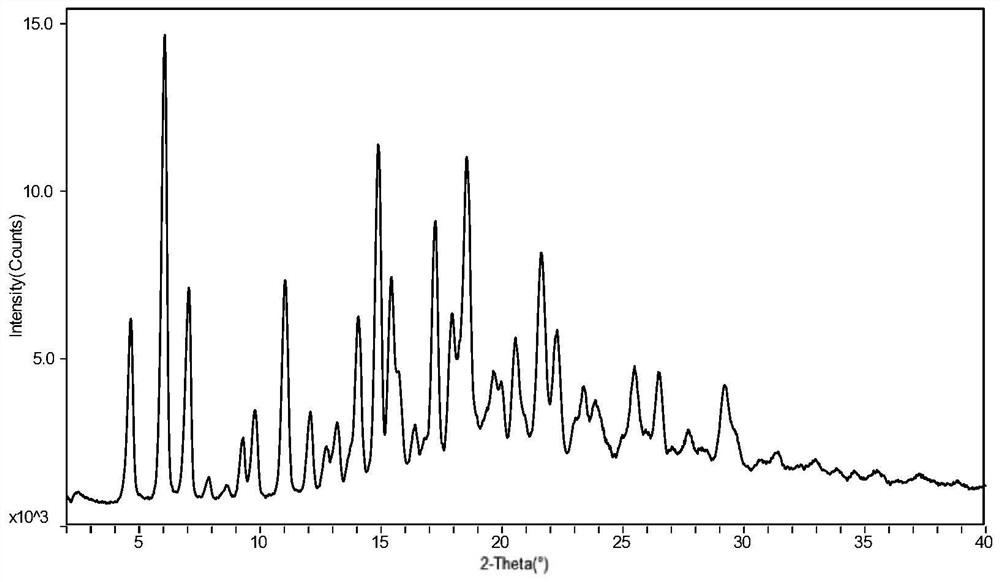
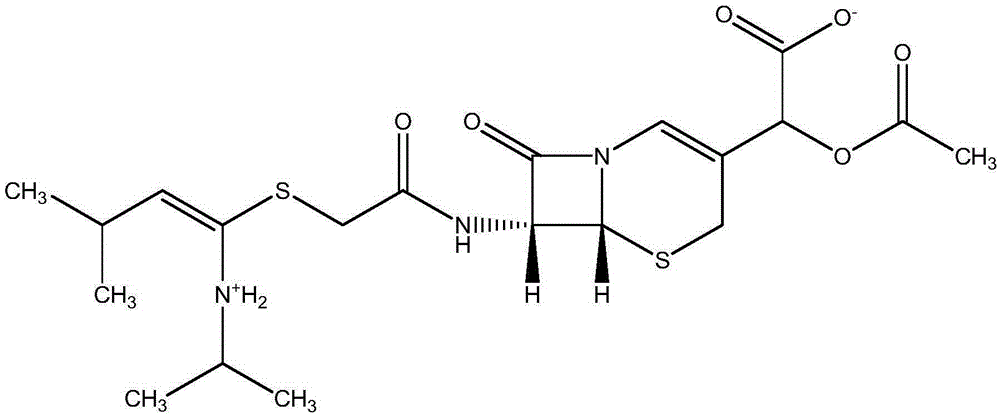
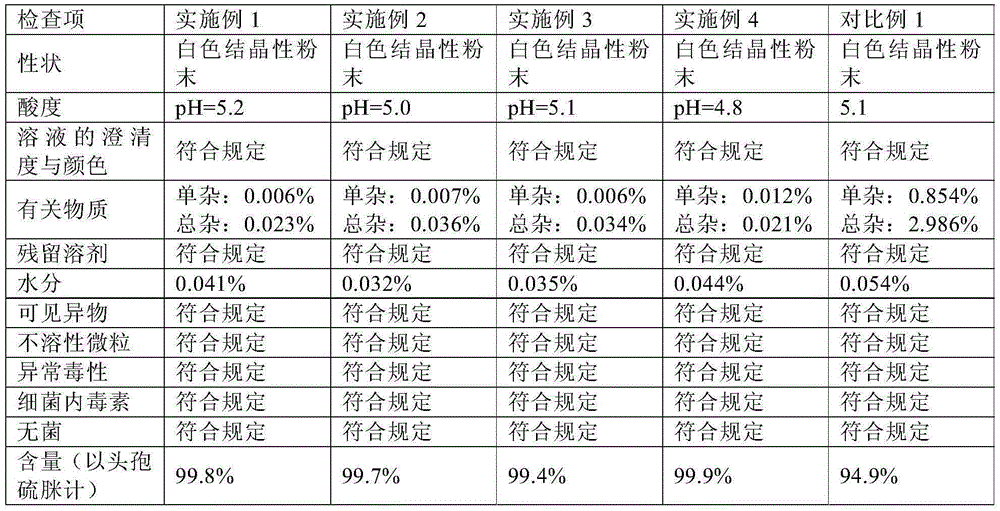
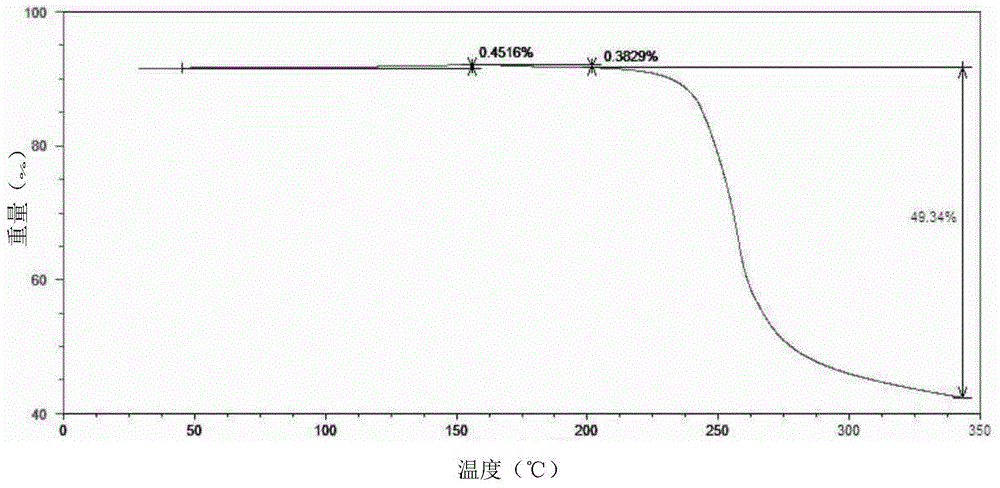
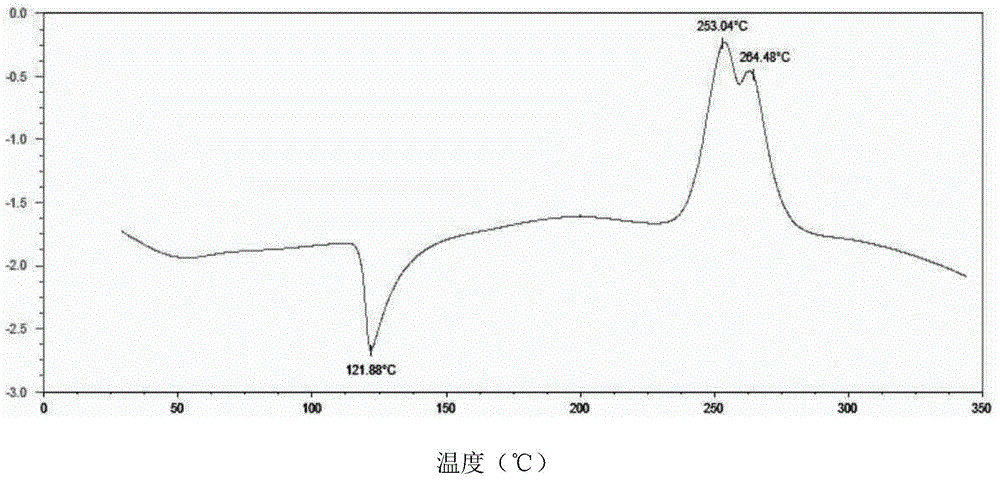
Preparation method of sofosbuvir crystal form 6
InactiveCN104829673AGood stabilitySimple crystallization processSugar derivativesSugar derivatives preparationSolventSofosbuvir
The invention discloses a novel crystallization method for preparing a sofosbuvir crystal form 6. Compared with an original preparation method of the crystal form 6, the novel crystallization method disclosed by the invention has the advantages that the purification effect is good, products are easy to dry, an environment-friendly effect is achieved and the like since sofosbuvir is prepared from a solution containing various organic mixed solvents through cooling crystallization.
Owner:CHARM PHARMATECH NANJING
Novel industrial crystallizing technology for cefuroxime sodium
ActiveCN104961749APromote enrichmentSimple crystallization processPowder deliverySolvent extractionPhysical chemistryCefuroxime Sodium
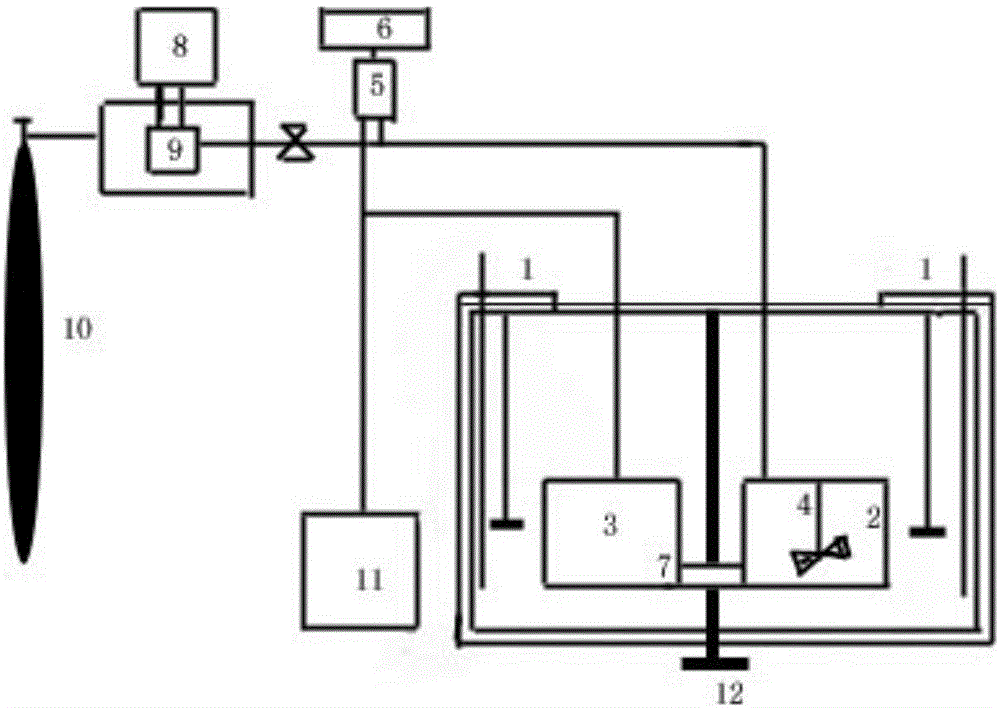
The invention discloses novel industrial crystallizing technology for cefuroxime sodium. Recrystallizing of cefuroxime sodium is realized by adopting a mode combining supercritical fluid extraction technology with conventional crystallizing technology. In a crystallizing system, the processes of extracting, adsorbing, crystallizing and drying are completed under specific temperature and pressure conditions and under joint action of supercritical fluid, solvent, an extraction pool and a crystallization pool to realize recrystallizing of cefuroxime sodium. The novel industrial crystallizing technology is high in separation efficiency and few in impurity, and quality of cefuroxime sodium is improved greatly.
Owner:HAINAN LINGKANG PHARMA CO LTD +1
2,2-dimethoxy-1,2-di[4-(METH)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition
The invention provides 2,2-dimethoxy-1,2-di-[4-(meth)acryloyloxy]phenylethane-1-one represented by the following formula (A):(wherein R1 and R2, which may be identical to or different from each other, each represent a hydrogen atom or a methyl group).
Owner:TOYO GOSEI IND CO
Preparation method of sofosbuvir crystal 6
InactiveCN106496295ASimple crystallization processHigh yieldSugar derivativesOrganic chemistry methodsSolventChemistry
The invention provides a preparation method of sofosbuvir crystal 6. The preparation method includes the steps: mixing a solution formed by sofosbuvir and an alcohol solvent, with water; crystallizing to obtain the sofosbuvir crystal 6. The preparation method is simple and efficient in crystallization process, high in yield and good in repeatability, the obtained crystal is good in stability and purity and meets requirement of oral solid preparations, the requirements of bulk pharmaceutical chemicals are met, and the preparation method is green and suitable for industrial production.
Owner:FUJIAN COSUNTER PHARMA
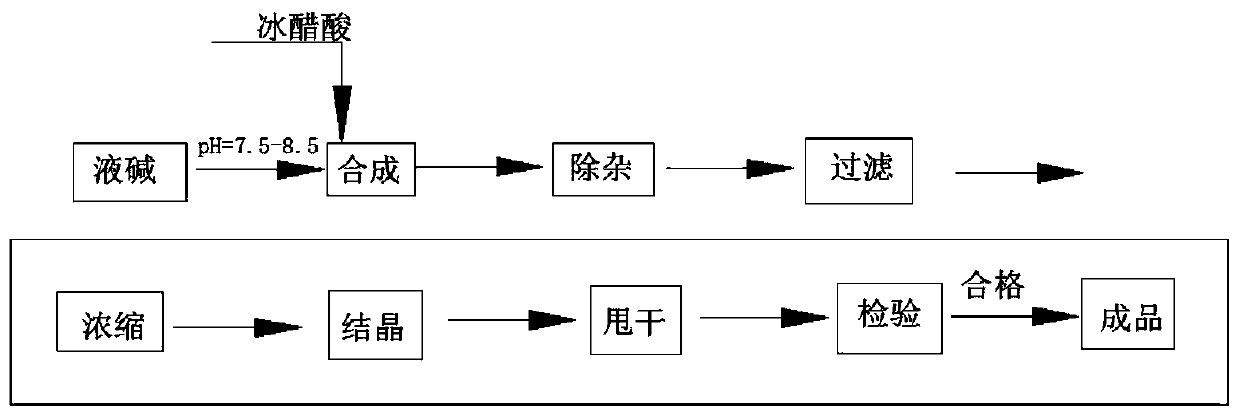
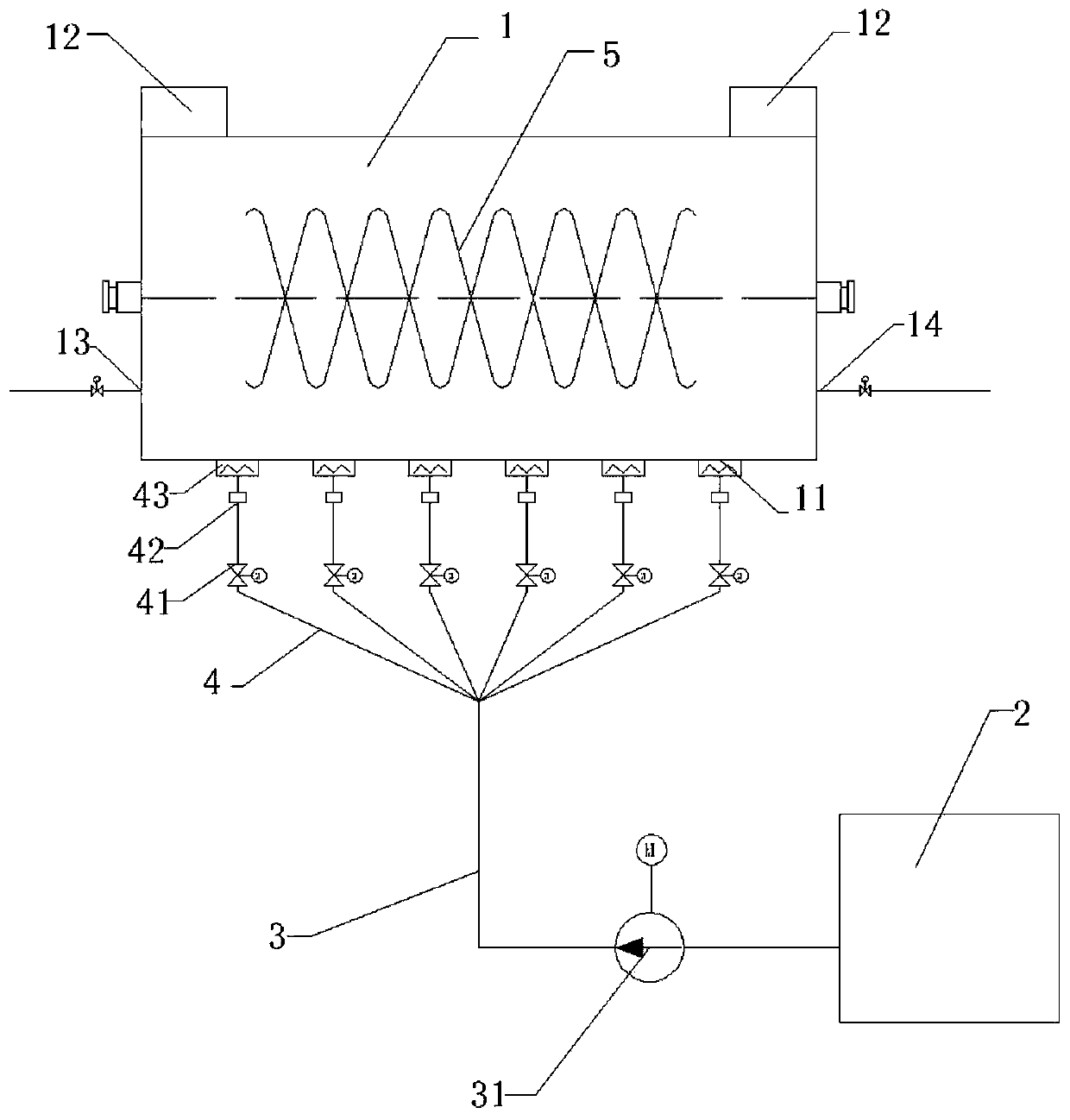
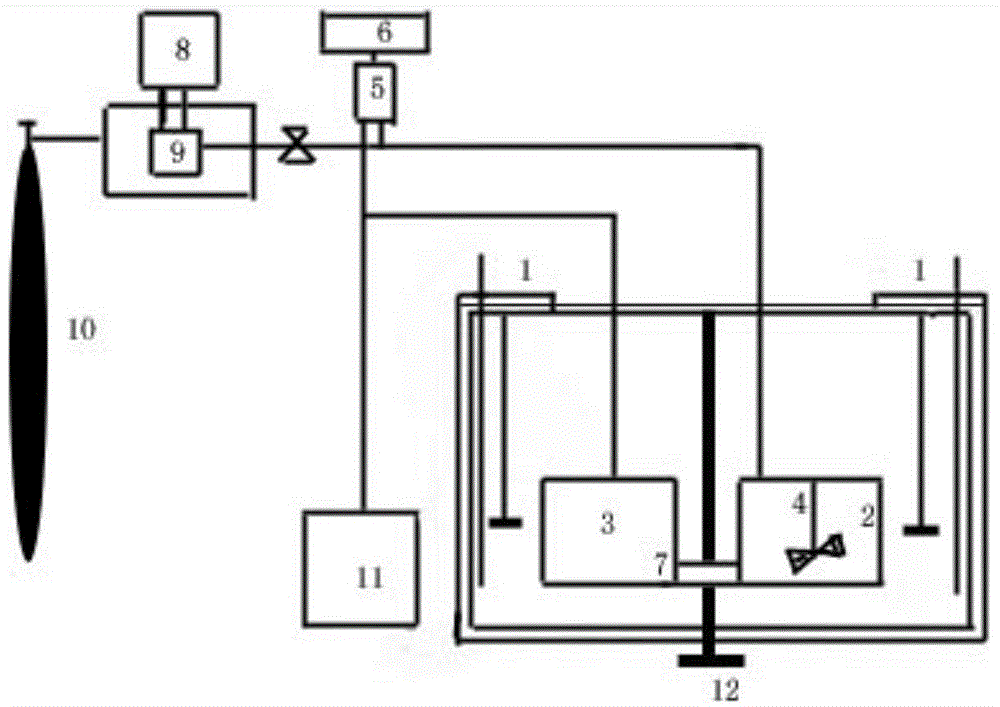
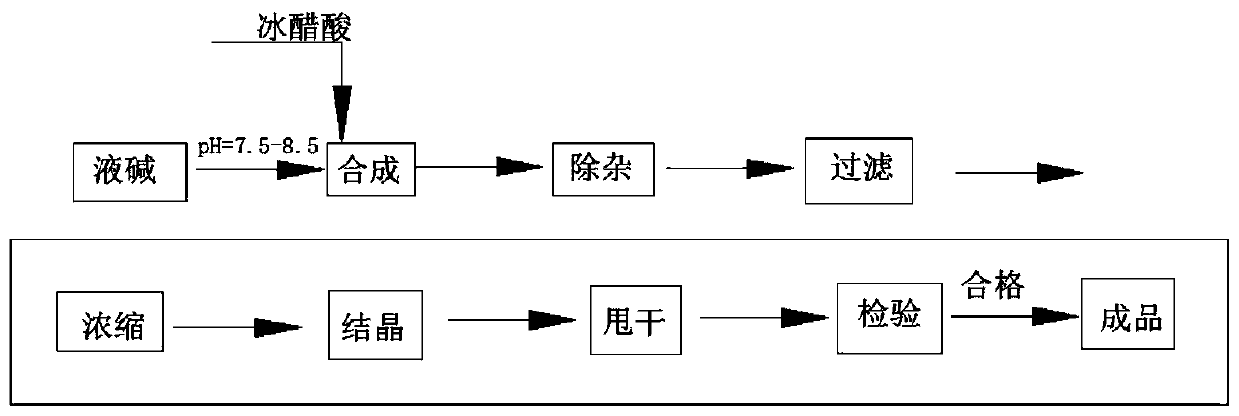
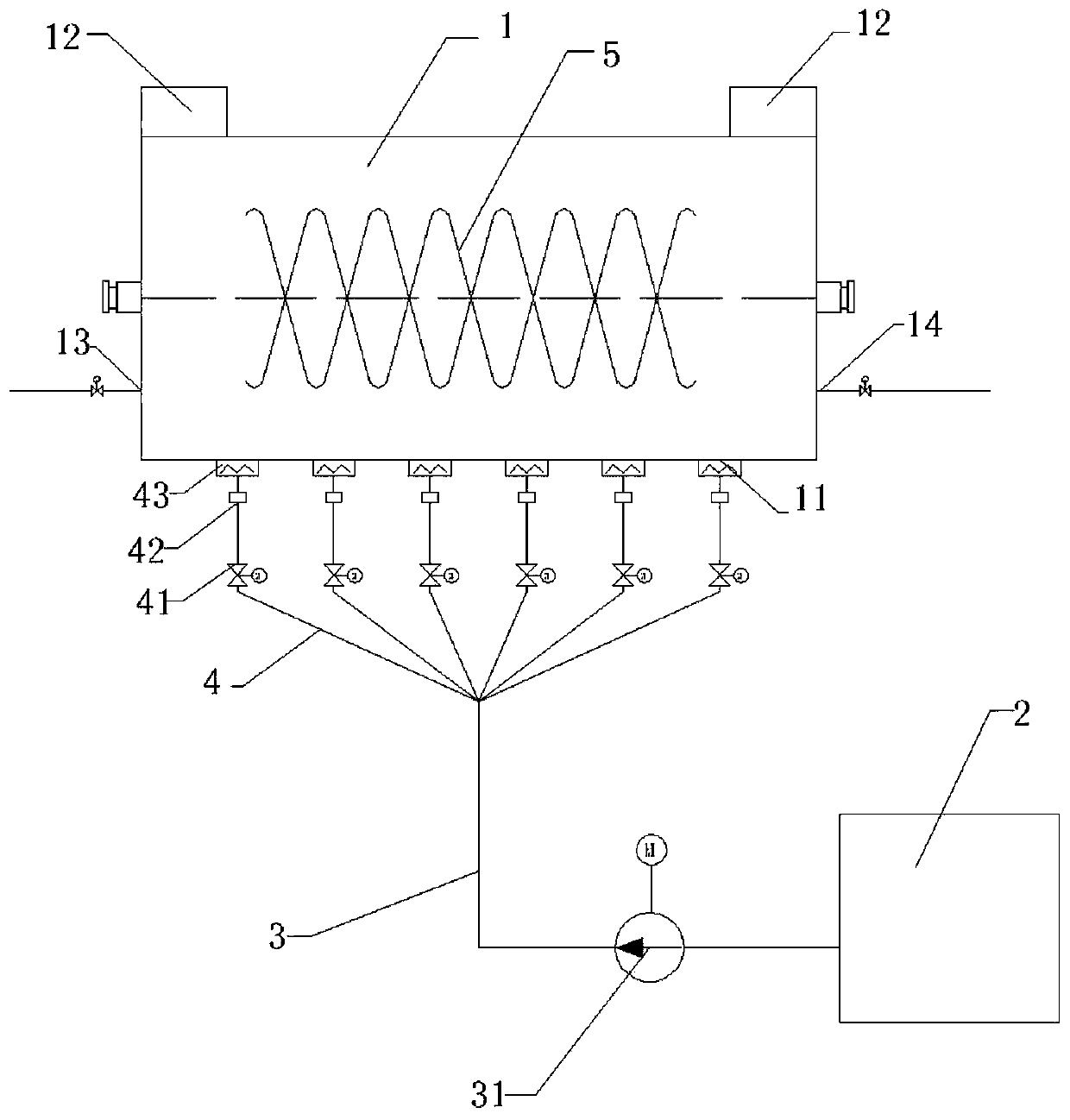
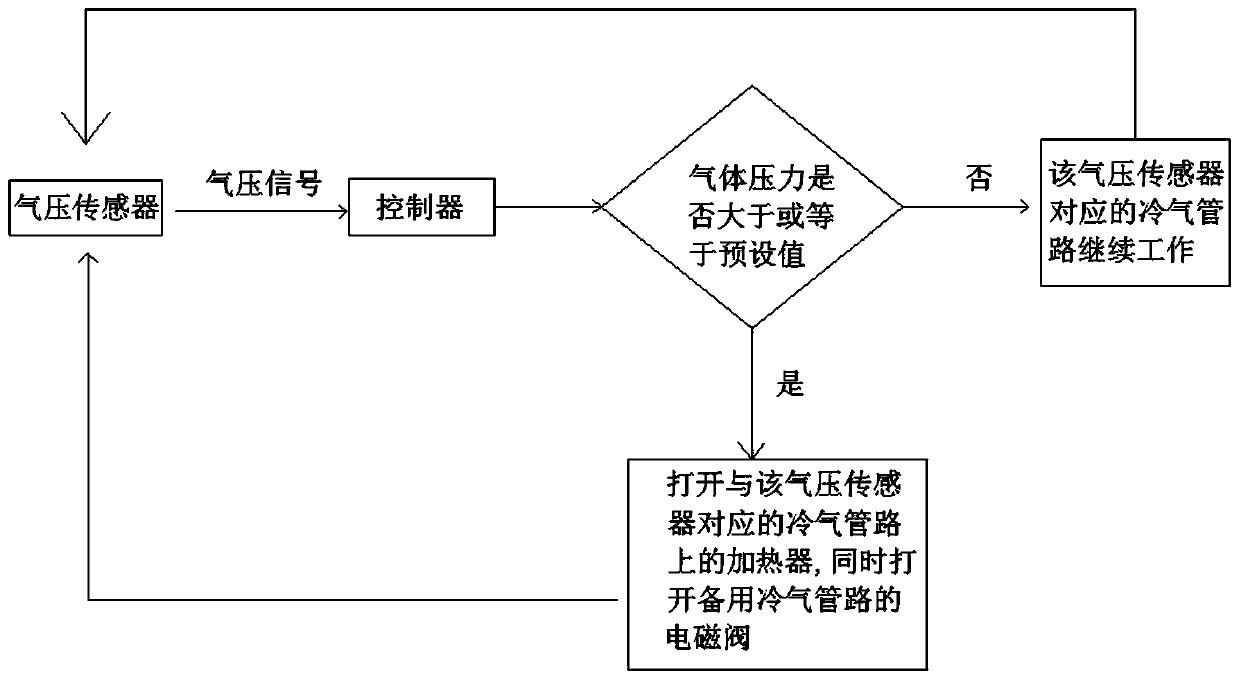
Sodium acetate production technology
ActiveCN109734582AHigh purityDiffusion fastSolution crystallizationCarboxylic acid salt preparationSodium acetateSodium acetrizoate
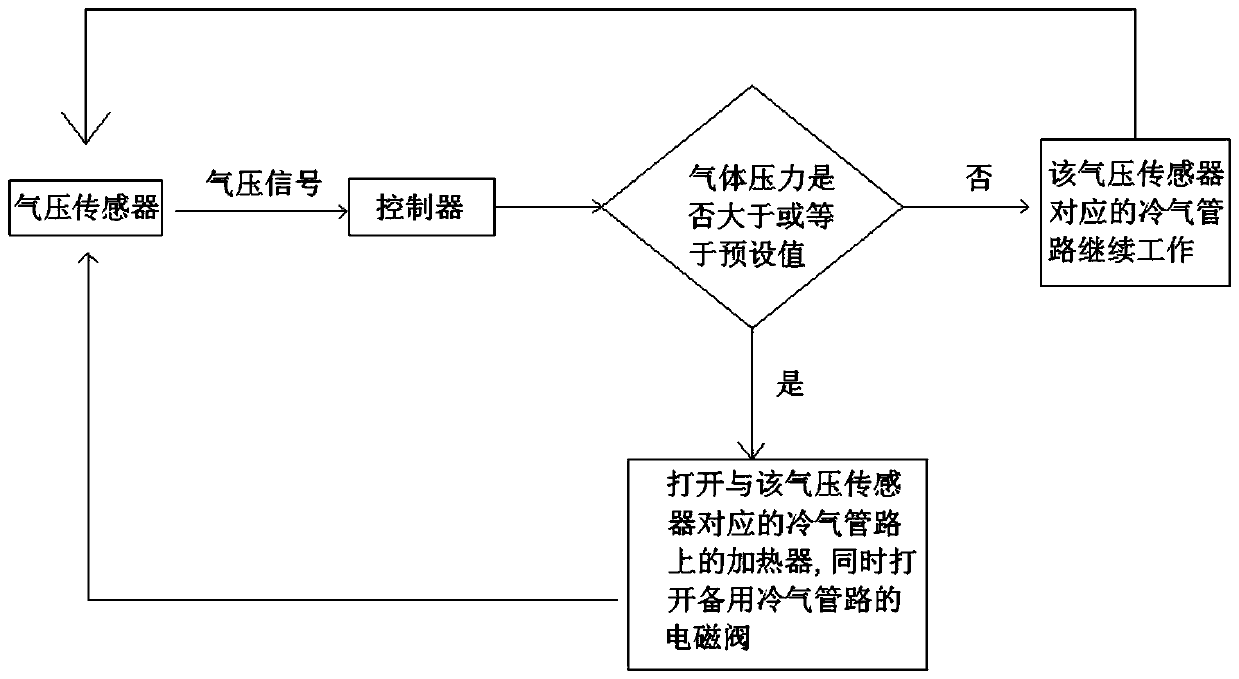
The invention relates to a sodium acetate production technology. The sodium acetate production technology comprises the following steps: synthesizing, purifying, filtering, concentrating, crystallizing, centrifuging and spin-drying, checking, and packaging. A crystallizing device of the sodium acetate production technology is different from a current jacket-type indirect contact cooling device. Acold gas in a lower temperature is used to be fed into concentrated liquid for cooling, diffusion of the cold gas and the liquid is rapid, and contact is uniform, so cooling efficiency is better. Through installing multiple cold gas pipelines, a heater and an air pressure sensor, a problem of blocking and coagulating an air outlet can be rapidly detected and solved, so a crystallizing flow is simpler, and easy to automatically operate and control. An obtained sodium acetate trihydrate product is higher in purity, and reaches a detecting requirement.
Owner:台山市新宁制药有限公司
Method for preparing crystalline form 6 of Sofosbuvir
The invention discloses a method for preparing crystalline form 6 of Sofosbuvir. The method comprises the following steps: 1) adding Sofosbuvir in a mixed solvent of an alcohols solvent and water, heating the material and dissolving the material; 2) cooling the solution obtained in the step 1) for crystallization; and 3) filtering the solid after crystallization and drying the solid to obtain crystalline form 6 of Sofosbuvir. Compared with the original preparation method of crystalline form 6, the crystallization technology of the present invention is used for preparing crystalline form 6 of Sofosbuvir, and the method has the advantages of high yield, loose product, easy drying, little application amount of the solvent, environmental protection and simple operation.
Owner:SHANGHAI SHYNDEC PHARMA HAIMEN CO LTD +1
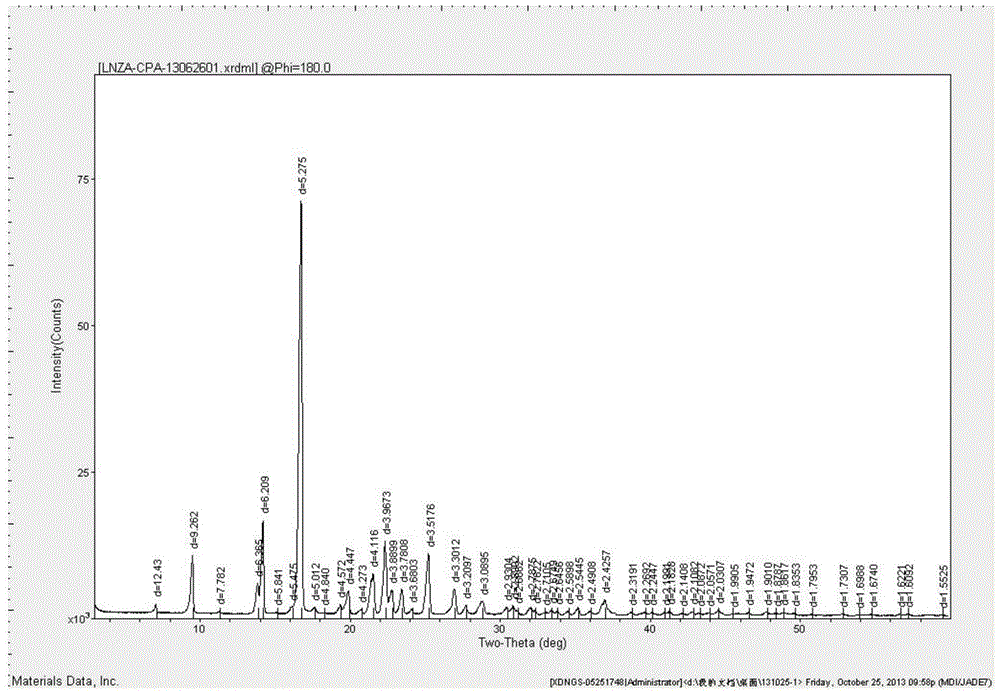
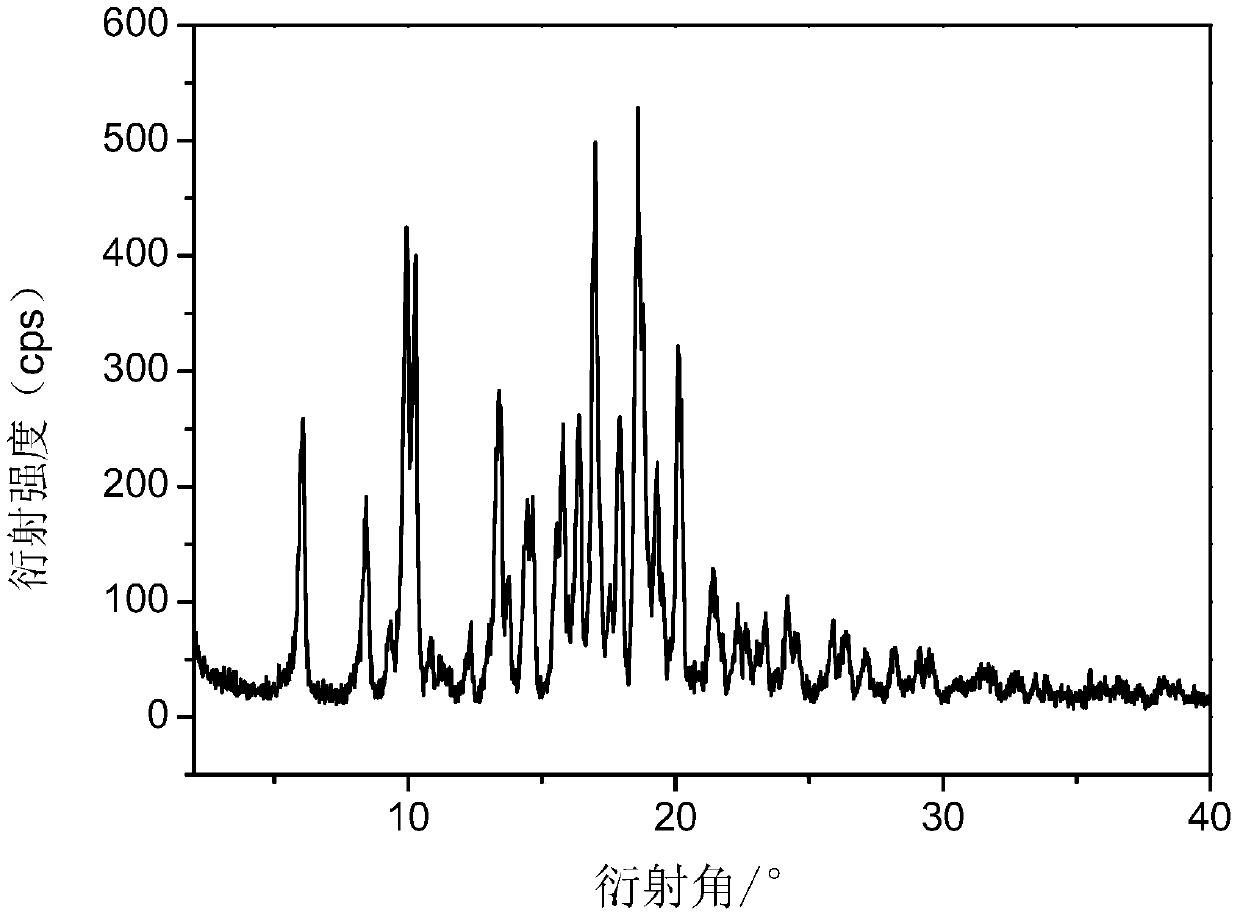
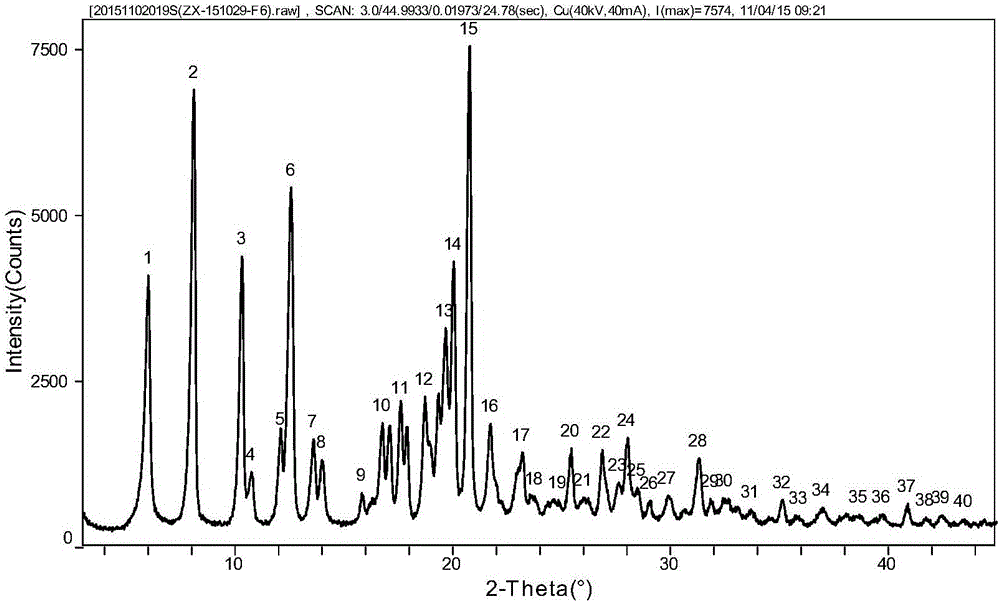
Tildipirosin 1,4-dioxane solvate and preparation method thereof
ActiveCN106008629AUniform particle size distributionPrevent coalescenceSugar derivativesOrganic chemistry methodsEnergy consumptionPowder diffraction
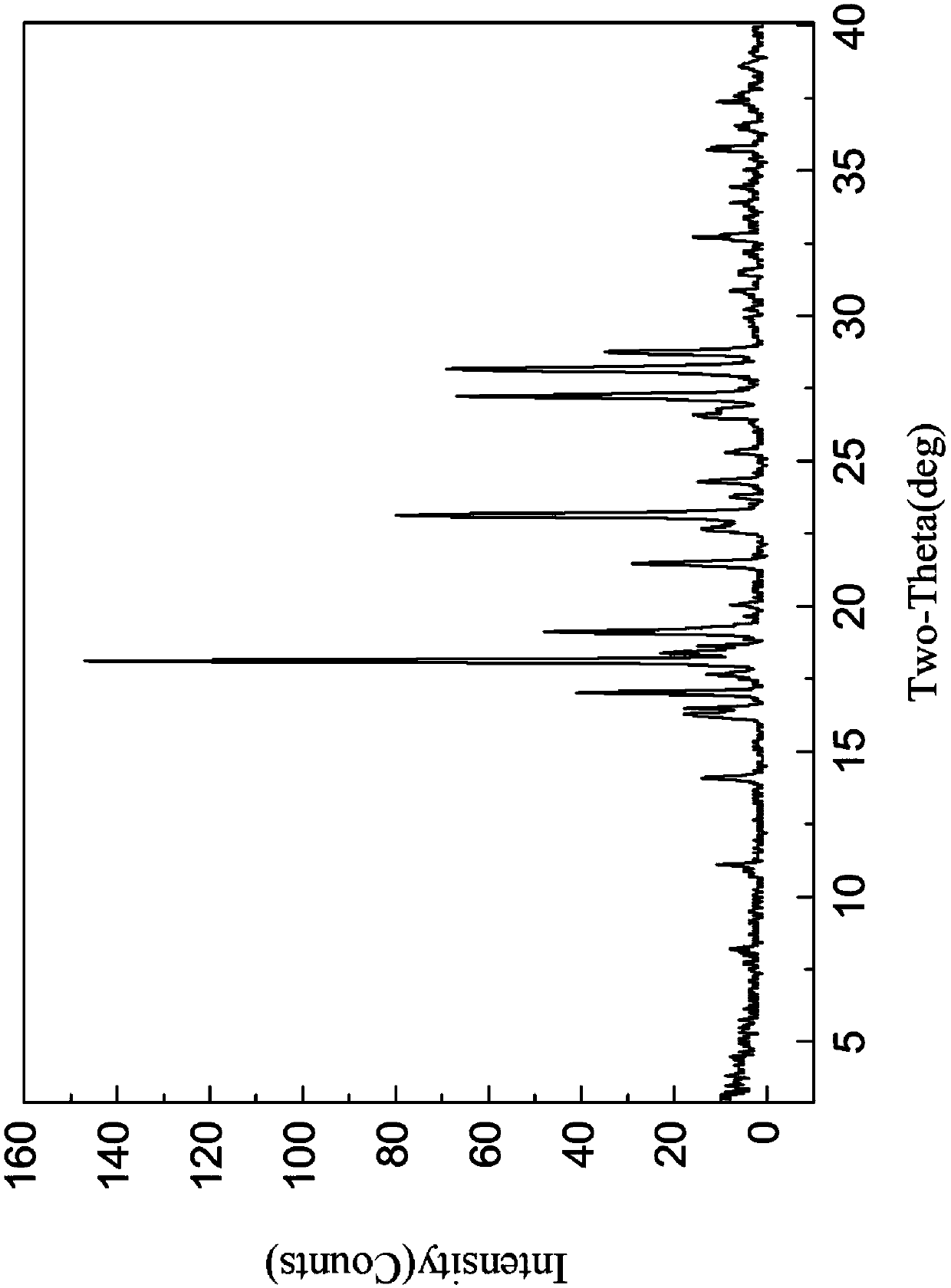
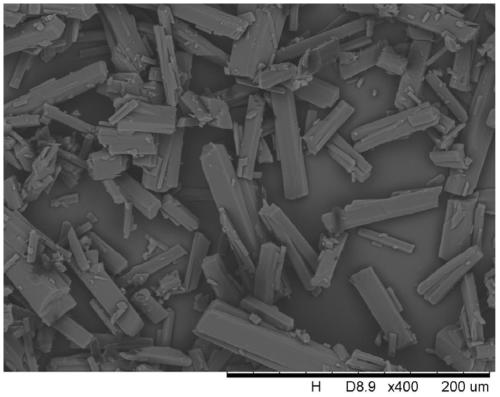
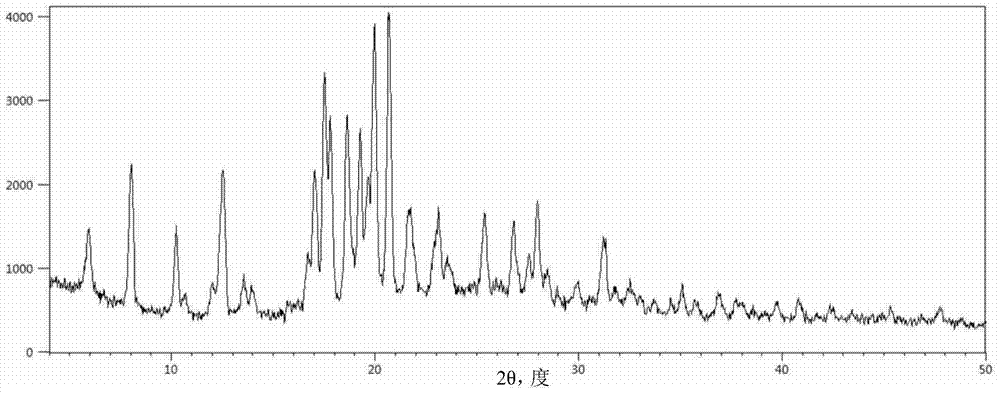
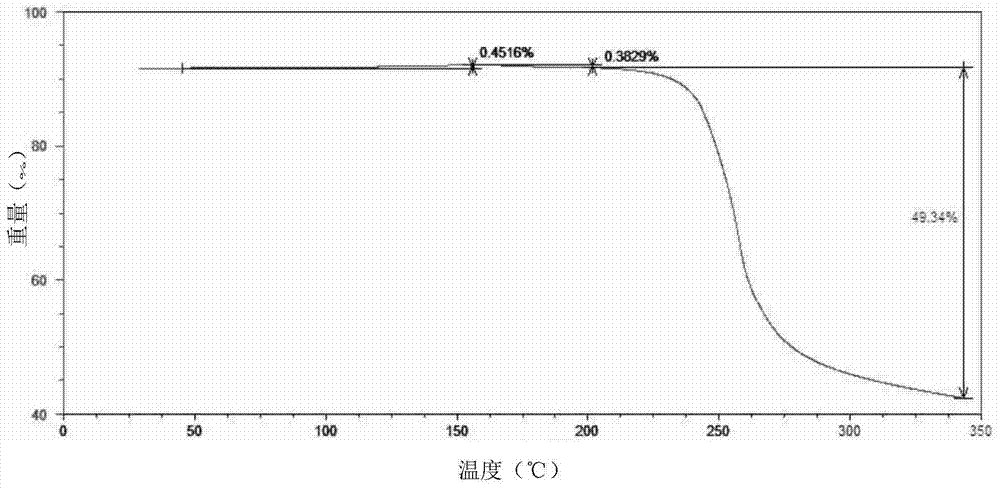
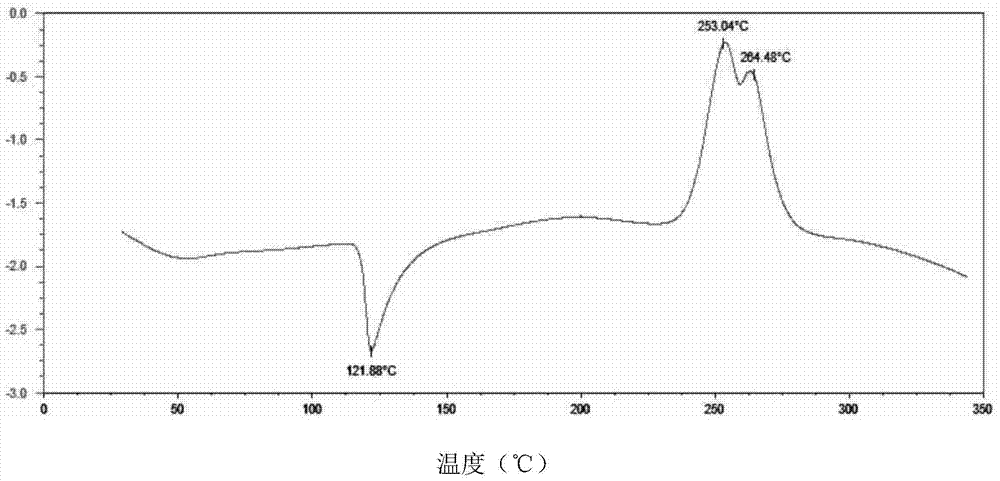
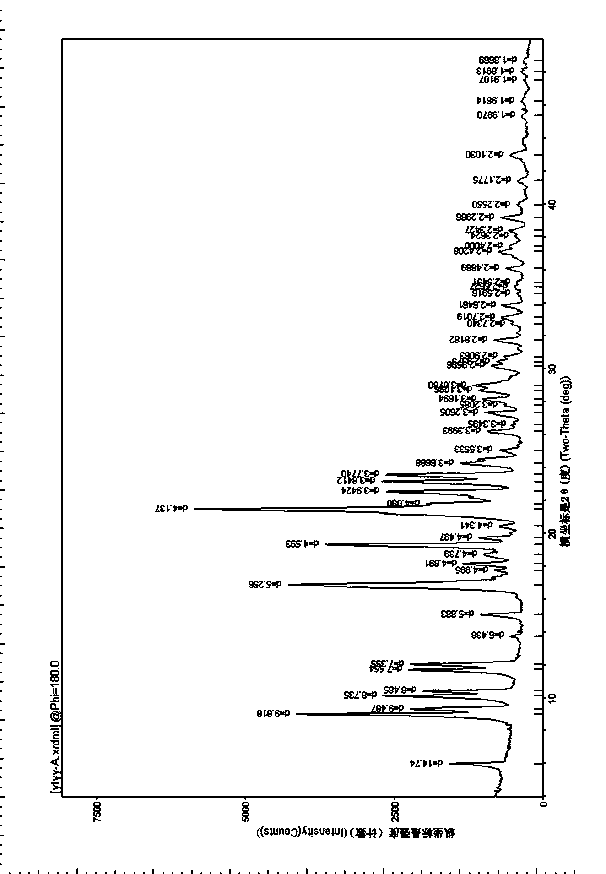
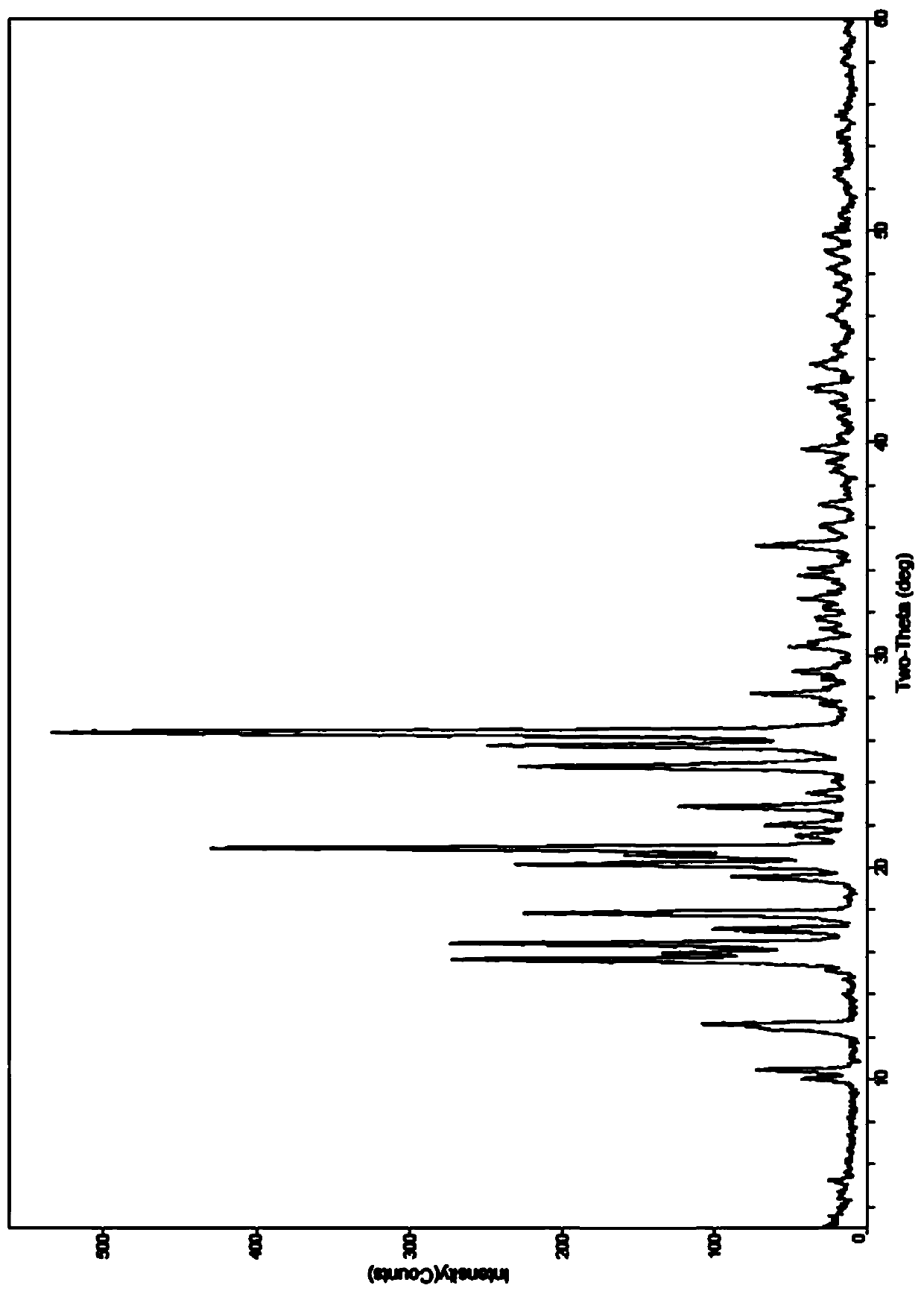
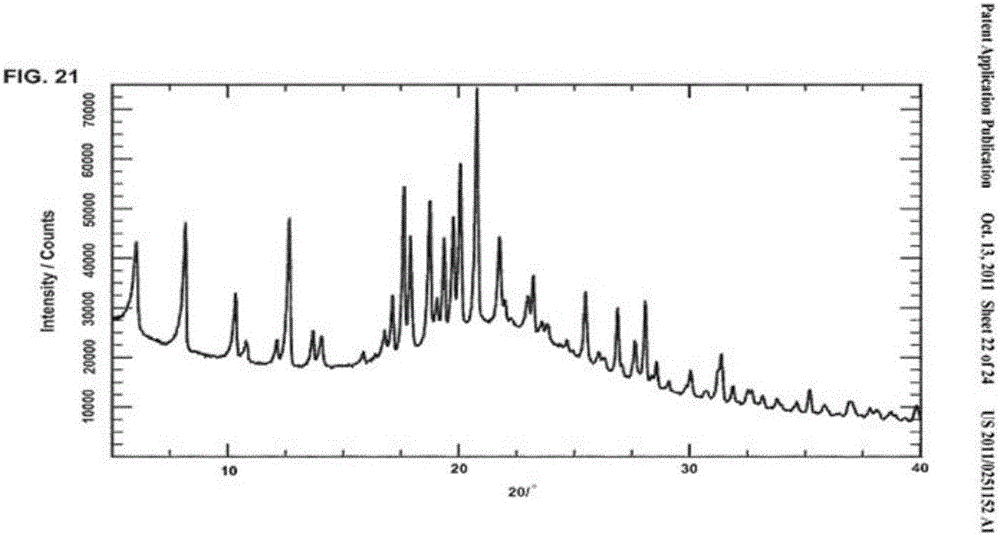
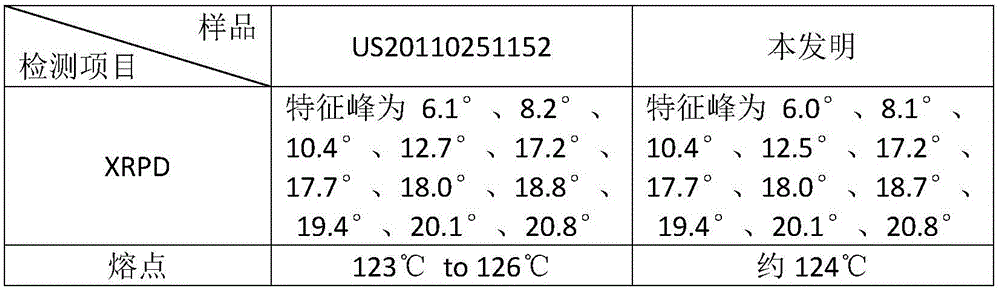
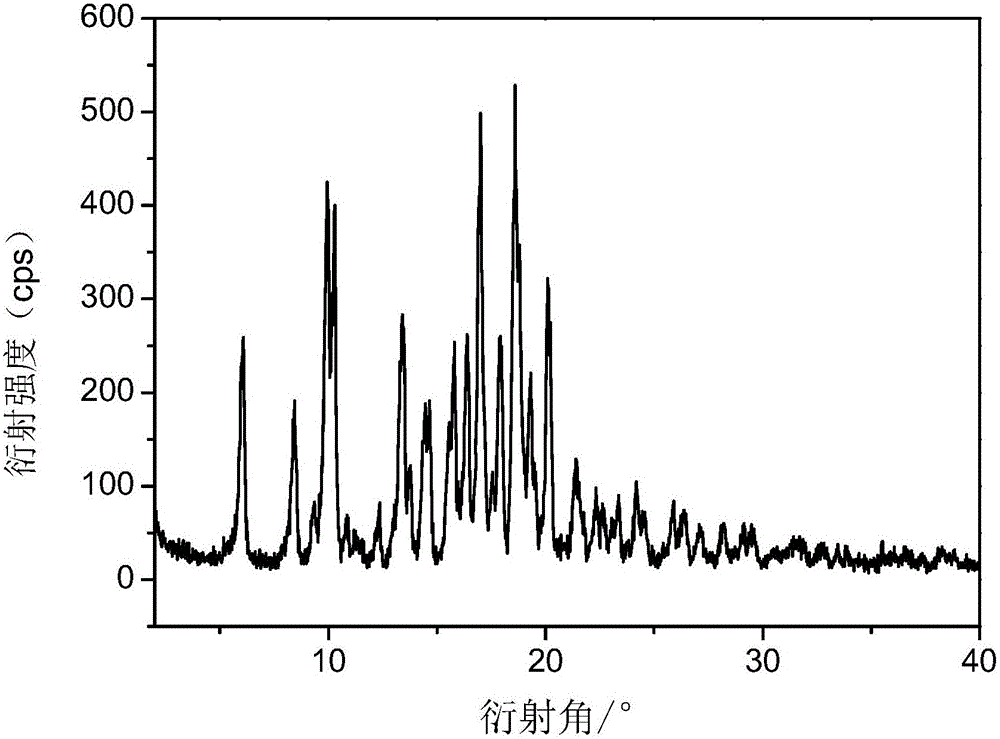
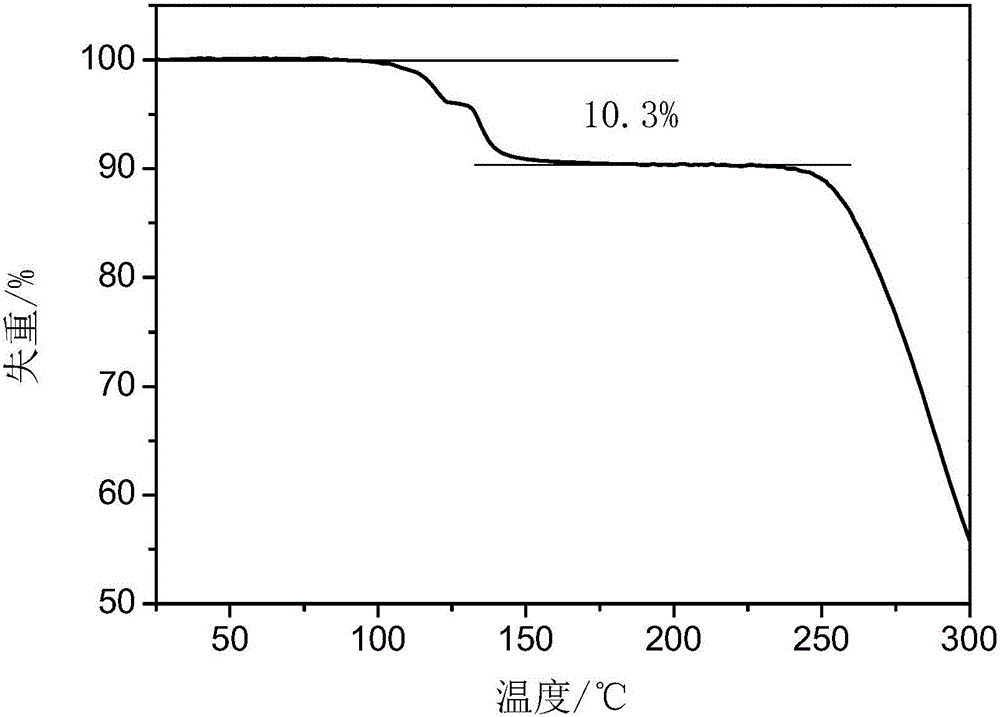
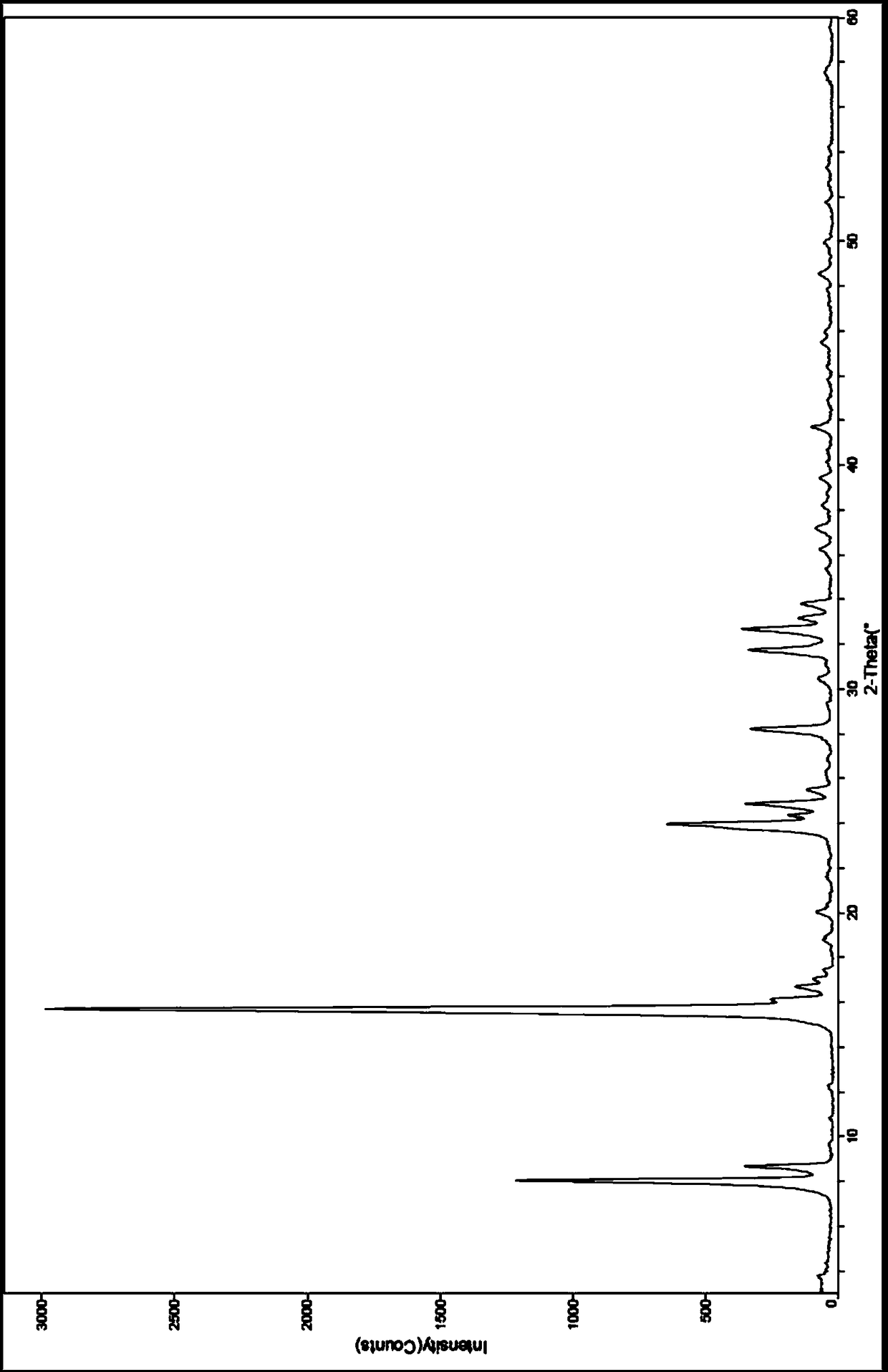
The invention provides a tildipirosin 1,4-dioxane solvate and a preparation method thereof. Characteristic peaks occur in an X-ray powder diffraction pattern represented by 2theta when 2theta is equal to 6.06+ / -0.2, 8.42+ / -0.2, 9.34+ / -0.2, 9.94+ / -0.2, 10.28+ / -0.2, 12.34+ / -0.2, 13.44+ / -0.2, 13.74+ / -0.2, 14.66+ / -0.2, 15.58+ / -0.2, 15.78+ / -0.2, 16.40+ / -0.2, 17.00+ / -0.2, 17.90+ / -0.2, 18.60+ / -0.2, 19.32+ / -0.2, 20.10+ / -0.2, 21.40+ / -0.2, 22.34+ / -0.2, 22.68+ / -0.2, 23.34+ / -0.2 and 24.18+ / -0.2. The preparation method employs a constant-temperature suspended crystal transformation process and is simple in process and low in energy consumption. The tildipirosin 1,4-dioxane solvate is in a rod shape and crystal is of a regular shape.
Owner:TIANJIN UNIV
Dapagliflozin crystal form and preparation method and purpose thereof
InactiveCN108516966AImprove solubilitySimple crystallization processOrganic active ingredientsMetabolism disorderDissolutionChemical stability
The invention relates to a preparation method and a purpose for a dapagliflozin.(S)-propylene glycol.hydrate crystal form D. The crystal form has excellent properties in terms of dissolution time, biology release, chemical stability and processing adaptability.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Novel industrial crystallizing technology for cefathiamidine
ActiveCN104926833ANo residual toxicityNot easy to decompose and destroyOrganic chemistry methodsBulk chemical productionSolventImpurity
The invention discloses a novel industrial crystallizing technology for cefathiamidine, Recrystallization of the cefathiamidine is achieved by adopting the mode of combining the supercritical fluid extraction technology and the traditional crystallizing technology. In the whole crystallizing system, the process of extraction, adsorption, crystallization and drying is completed under the joint effect of supercritical fluid, a solvent, an extraction pool and a crystallizing pool under the condition of specific temperature and pressure, and recrystallization of the cefathiamidine is achieved. The method is high in separation efficiency and product purity and few in impurities, and greatly improves the quality of preparation products.
Owner:HAINAN LINGKANG PHARMA CO LTD +1
2,2-dimethoxy-1,2-DI[4-(meth)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition
The invention provides 2,2-dimethoxy-1,2-di-[4-(meth)acryloyloxy]phenylethane-1-one represented by the following formula (A):(wherein R1 and R2, which may be identical to or different from each other, each represent a hydrogen atom or a methyl group).
Owner:TOYO GOSEI IND CO
Novel industrial crystallization technology for cefamandole nafate
ActiveCN104892637APromote enrichmentSimple crystallization processOrganic chemistryBulk chemical productionSolventImpurity
The invention discloses a novel industrial crystallization technology for cefamandole nafate. Recrystallization of the cefamandole nafate is realized through combination of a supercritical fluid extraction technology and a traditional crystallization technology. In a whole crystallization system, extraction, adsorption, crystallization and drying processes are finished under the coaction of a supercritical fluid, a solvent, an extraction pool and a crystallization pool under specific temperature and pressure conditions, and recrystallization of the cefamandole nafate is realized. According to the method, the separation efficiency is high, the product purity is high, few impurities exist, and the preparation product quality is greatly improved.
Owner:HAINAN LINGKANG PHARMA CO LTD +1
Method for separating out nafcillin acid crystal from nafcillin sodium water solution
The invention relates to a method for separating out nafcillin acid crystal from a nafcillin sodium aqueous solution, which comprises the following steps: adding one of ethyl acetate, butyl acetate, n-butanol, n-hexane or petroleum ether as an extracting agent into the nafcillin sodium aqueous solution, and dripping an acidifying agent into the solution to make the pH value to be between 1.5 and 2.0 so as to separate out the crystal; and separating out the crystal, washing the crystal by purified water to make the pH value of washing liquor to be 4.0, drying the crystal at a temperature of between 45 and 55 DEG C to ensure that the moisture is below 3 percent to obtain the nafcillin acid crystal. The nafcillin acid crystal separated out by the extracting agent has the advantages of good crystal form, easy filtration, easy drying, less residual solvent in a product, and good quality; and the content of nafcillin acid is more than 93 percent. The crystallization method has the advantages of simple crystallize process, single extracting agent, and easy reclamation.
Owner:朗致集团江西医药有限公司
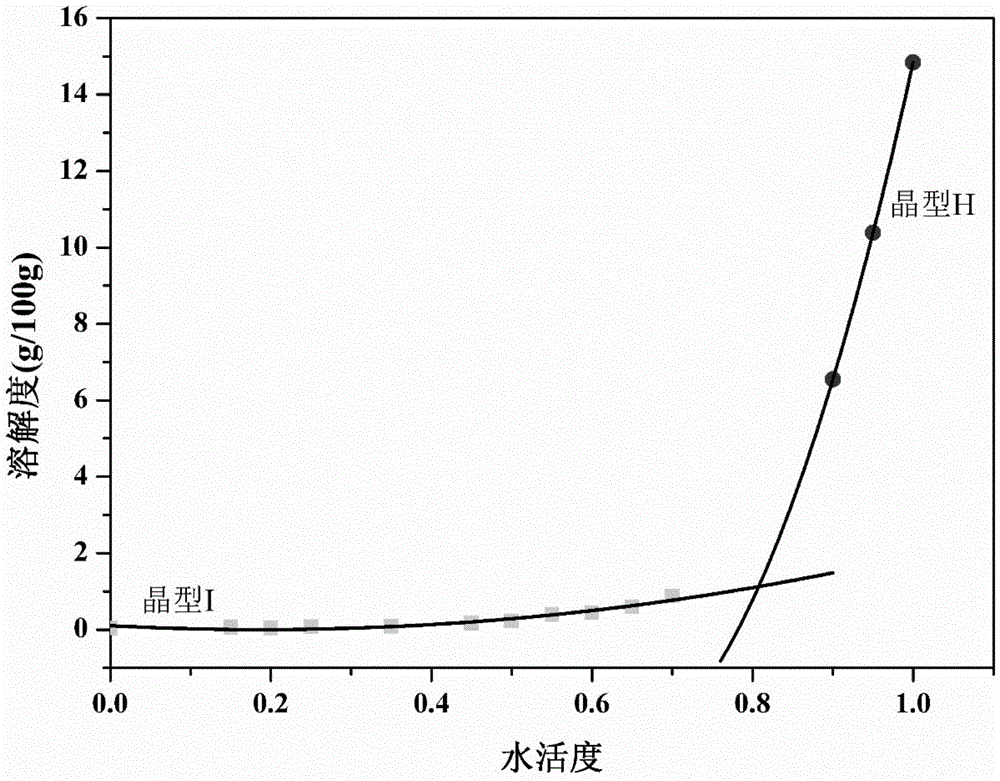
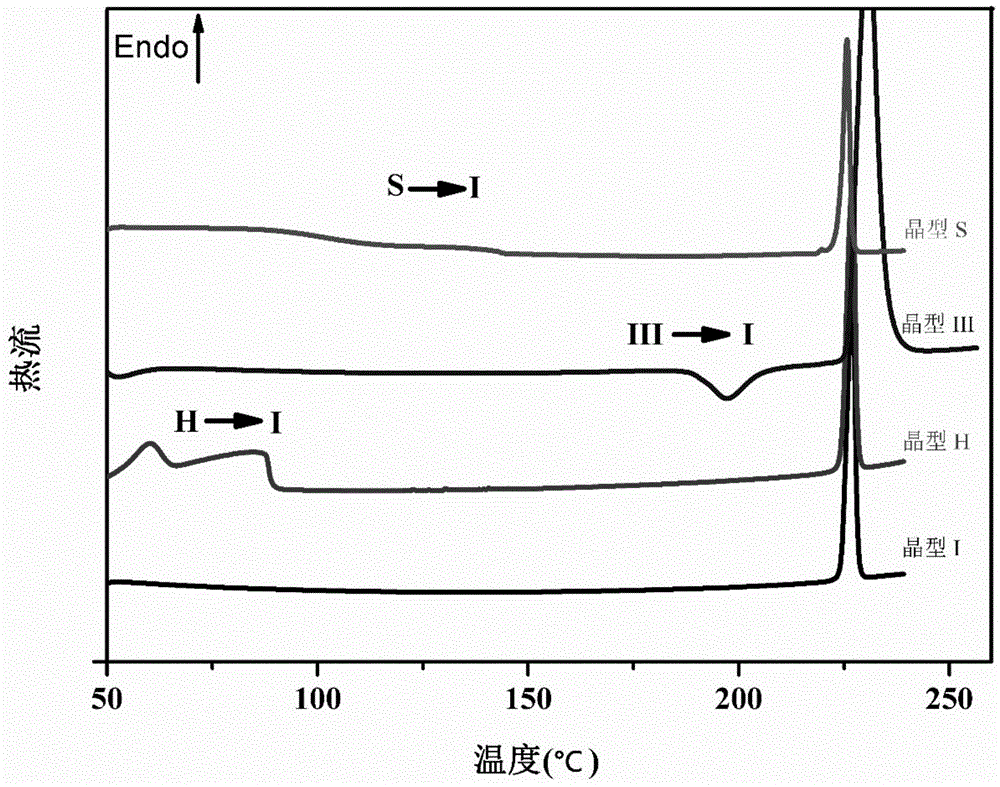
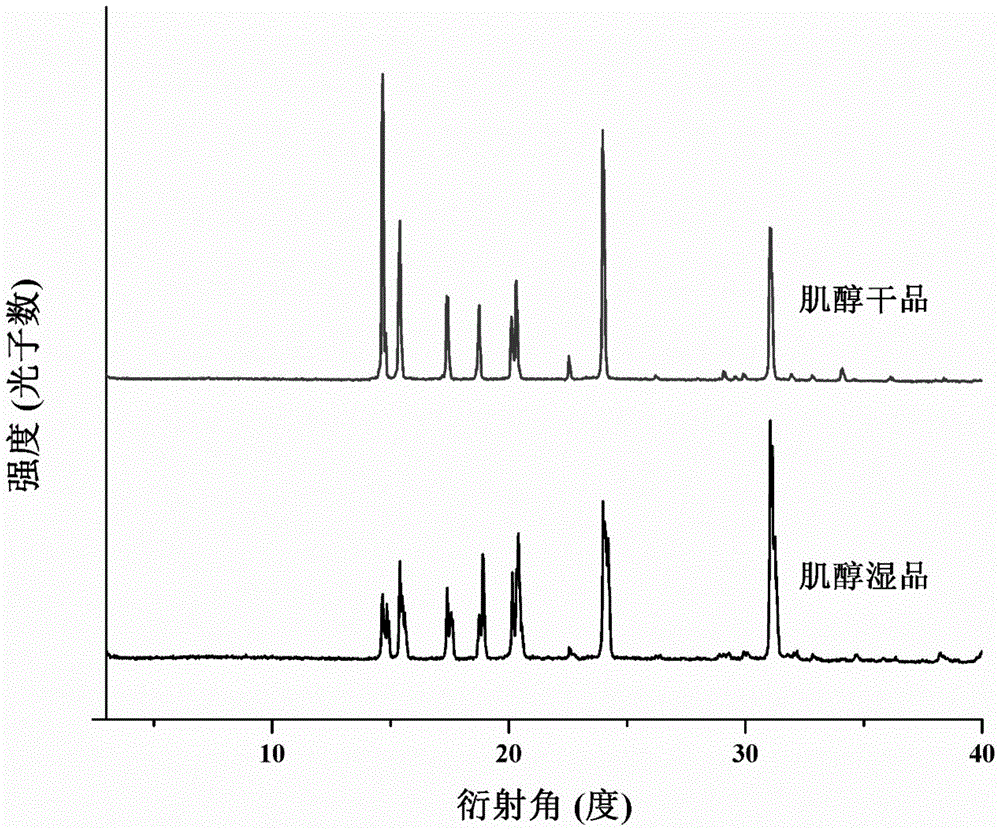
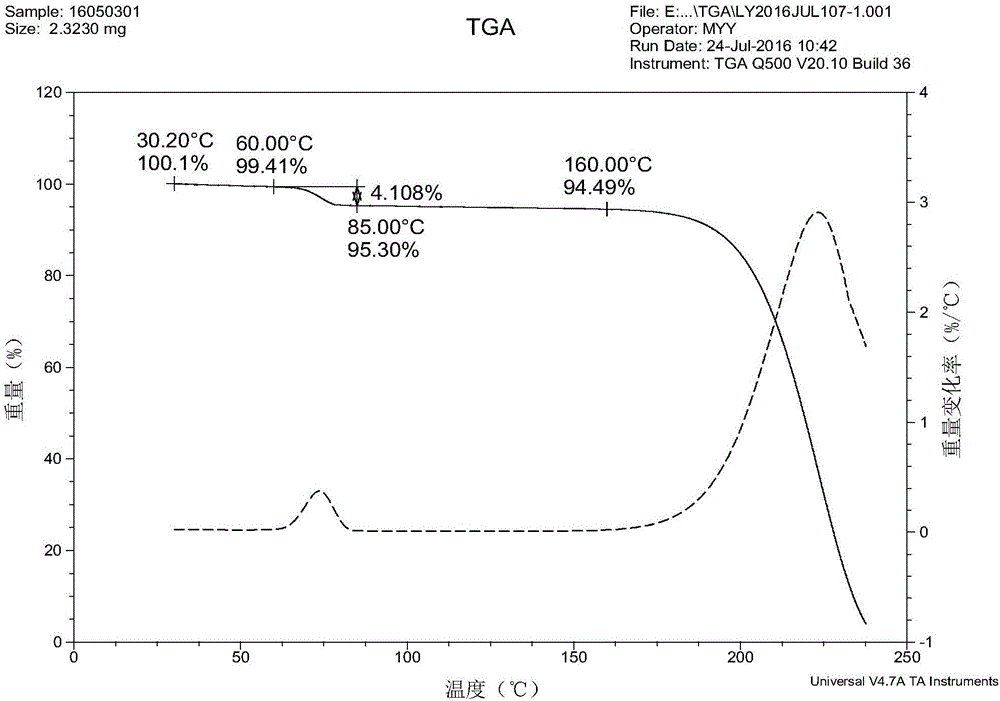
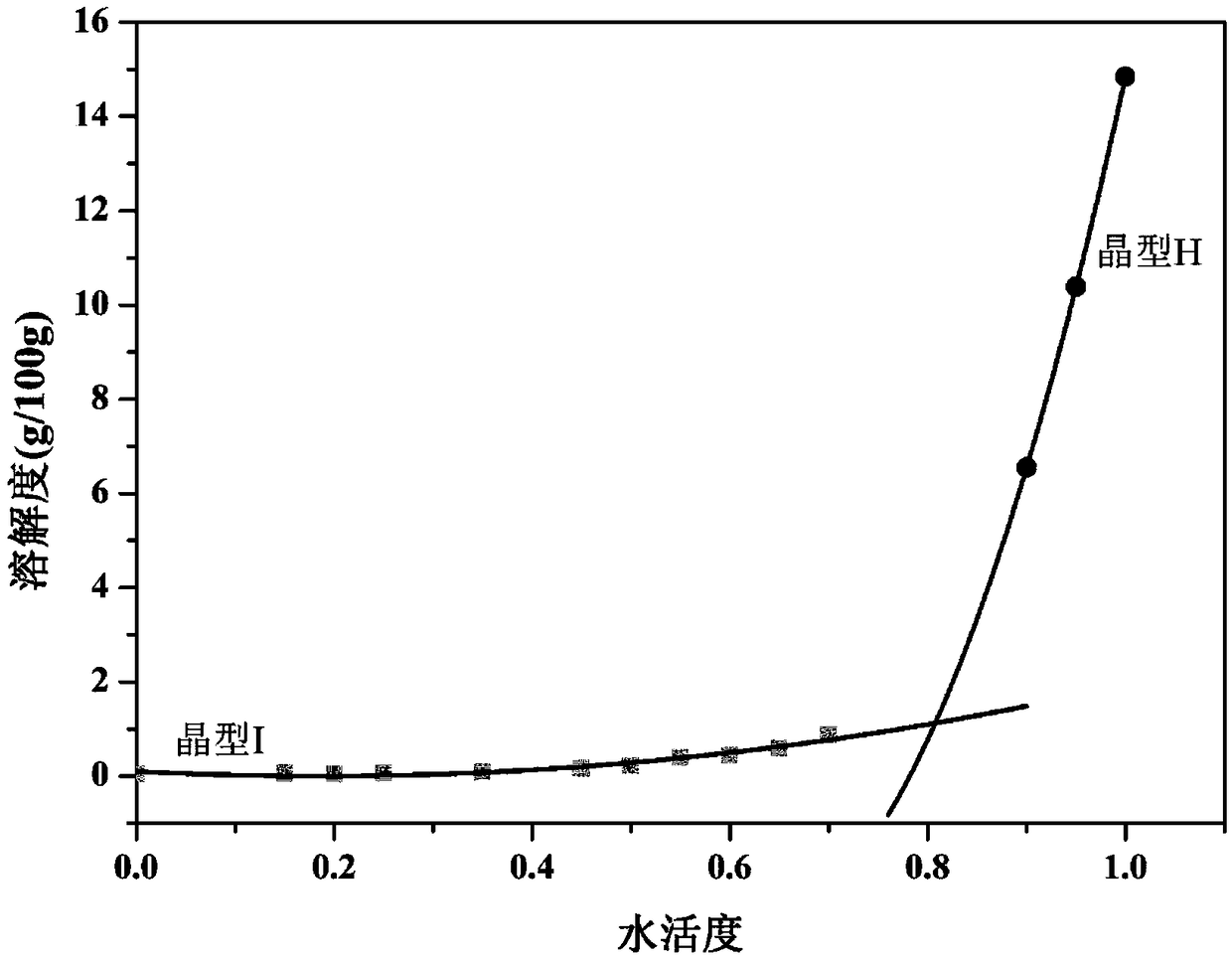
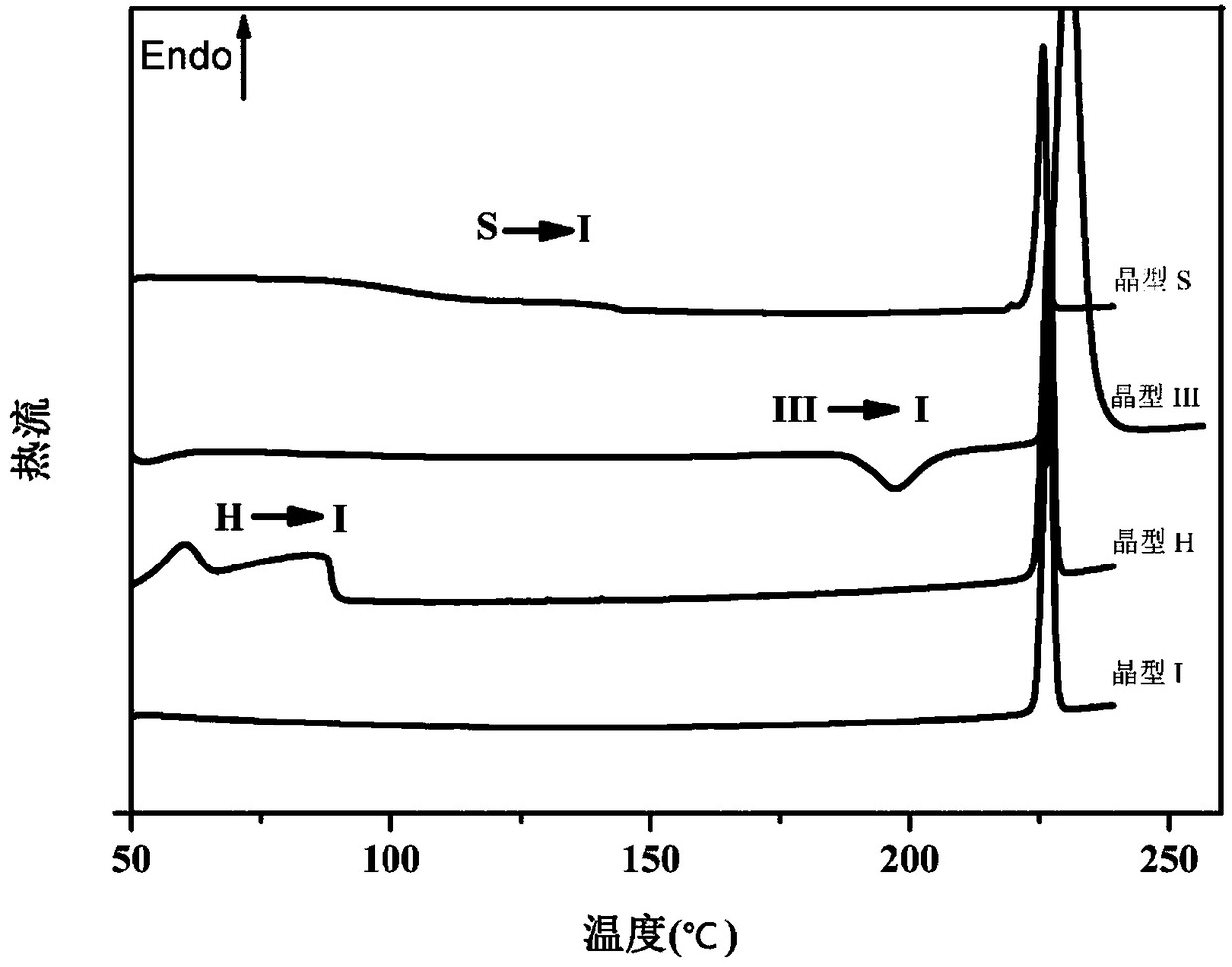
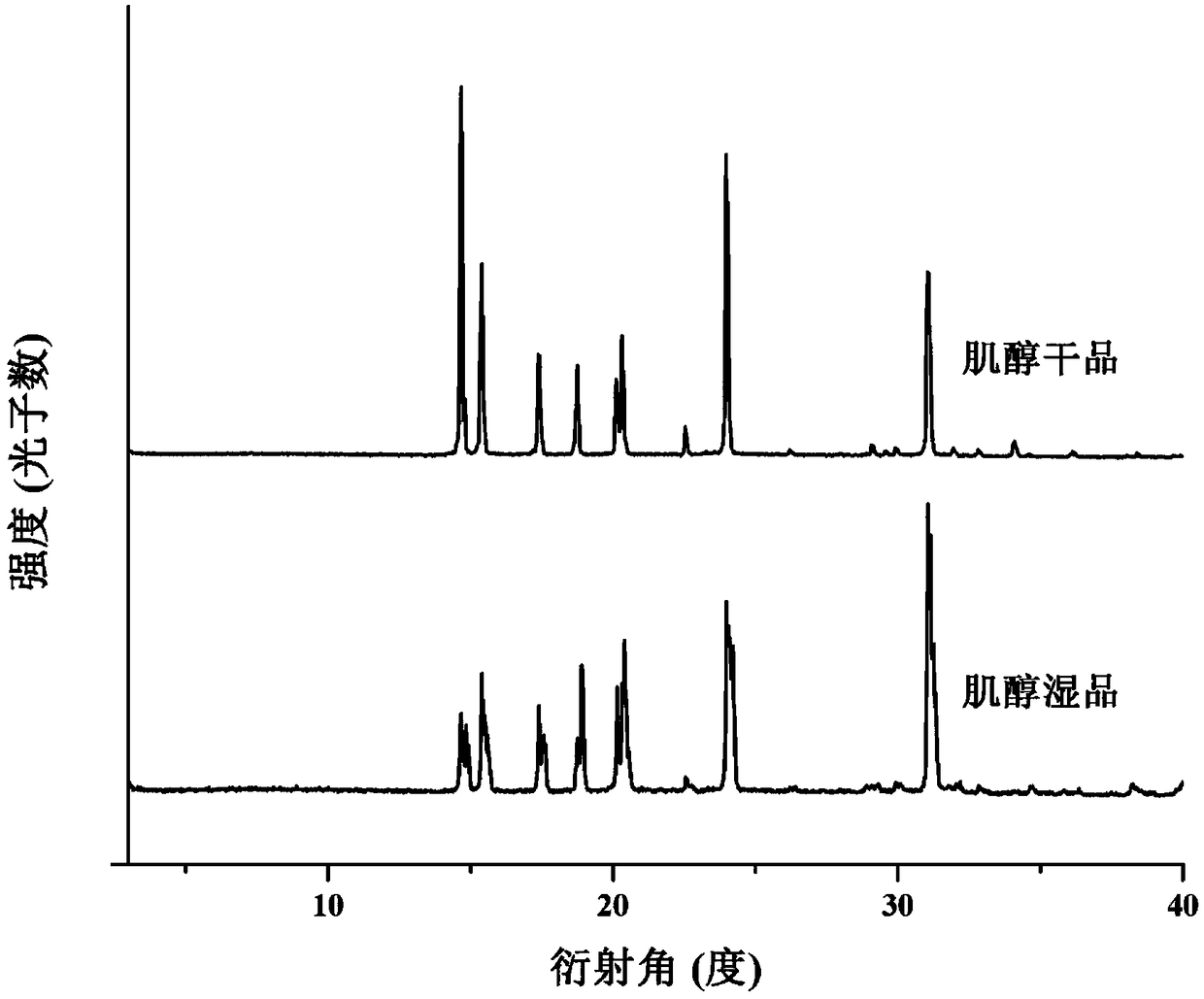
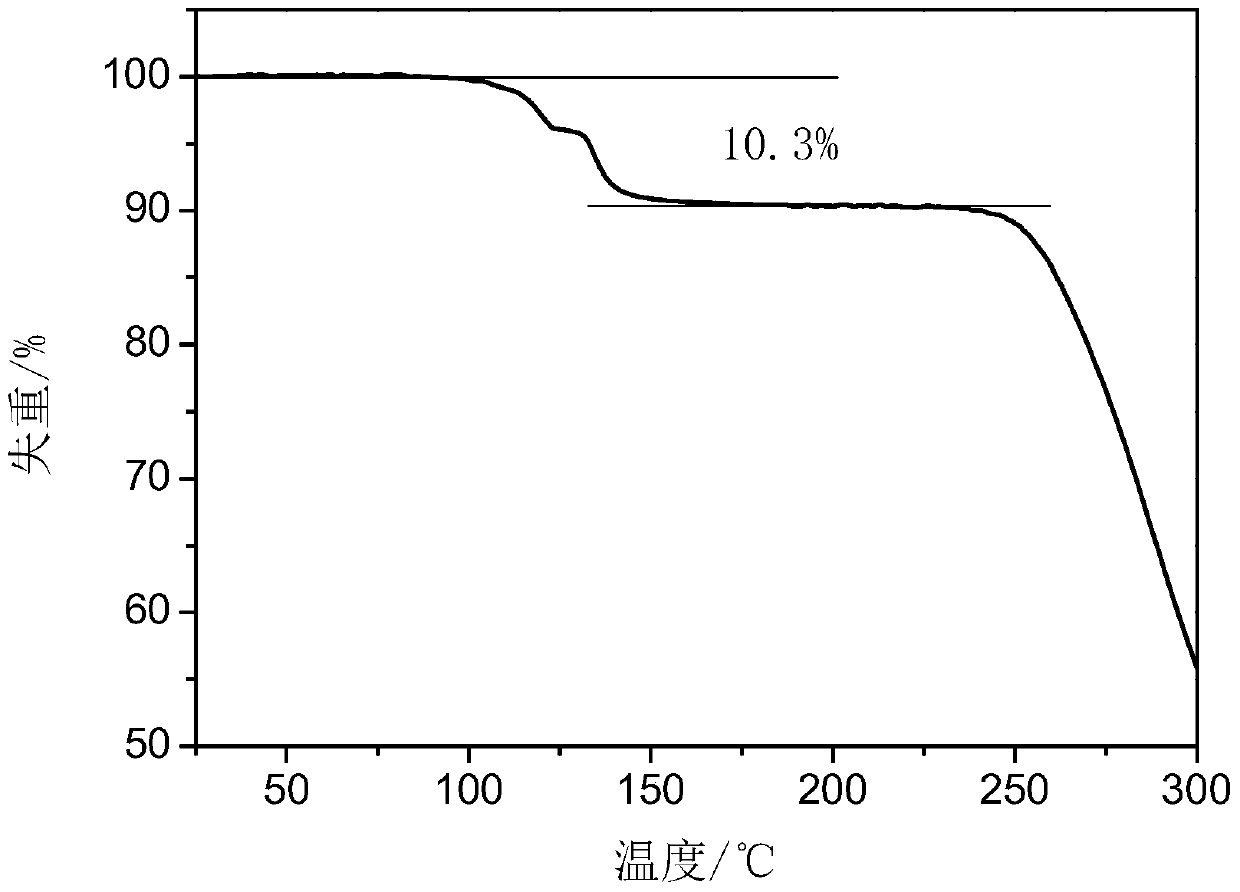
Crystallization process for preparing high-crystallinity myo-inositol with large particle size and application
ActiveCN105669376AAvoid it happening againAvoid chippingCosmetic preparationsHydroxy compound active ingredientsPhysical chemistryMoisture absorption
The invention belongs to the technical field of medicament crystallization processes, and discloses a crystallization process for preparing high-crystallinity myo-inositol with a large particle size. A product prepared by the crystallization process is columnar crystalline myo-inositol, a particle size thereof can be more than 100 microns, the product is high in crystallinity and uniform in particle size distribution, and the shortcomings of low content, high moisture absorption and caking rate, long suction filtration and drying time, poor flowability and the like of powdery myo-inositol prepared by the prior art are overcome. The crystallization process has the characteristics of process simplicity, low equipment requirements and the like, production of a dihydrate crystal form H in a process is avoided, caking and crystal breaking in a drying process are avoided, suction filtration and drying time is shortened, and process cost is reduced.
Owner:ZHUCHENG HAOTIAN PHARMA
Isavuconazole monohydrate crystal form and preparation method thereof
ActiveCN106749221ASimple crystallization processEasy to operateOrganic chemistry methodsDithiophosphoric acidX-ray
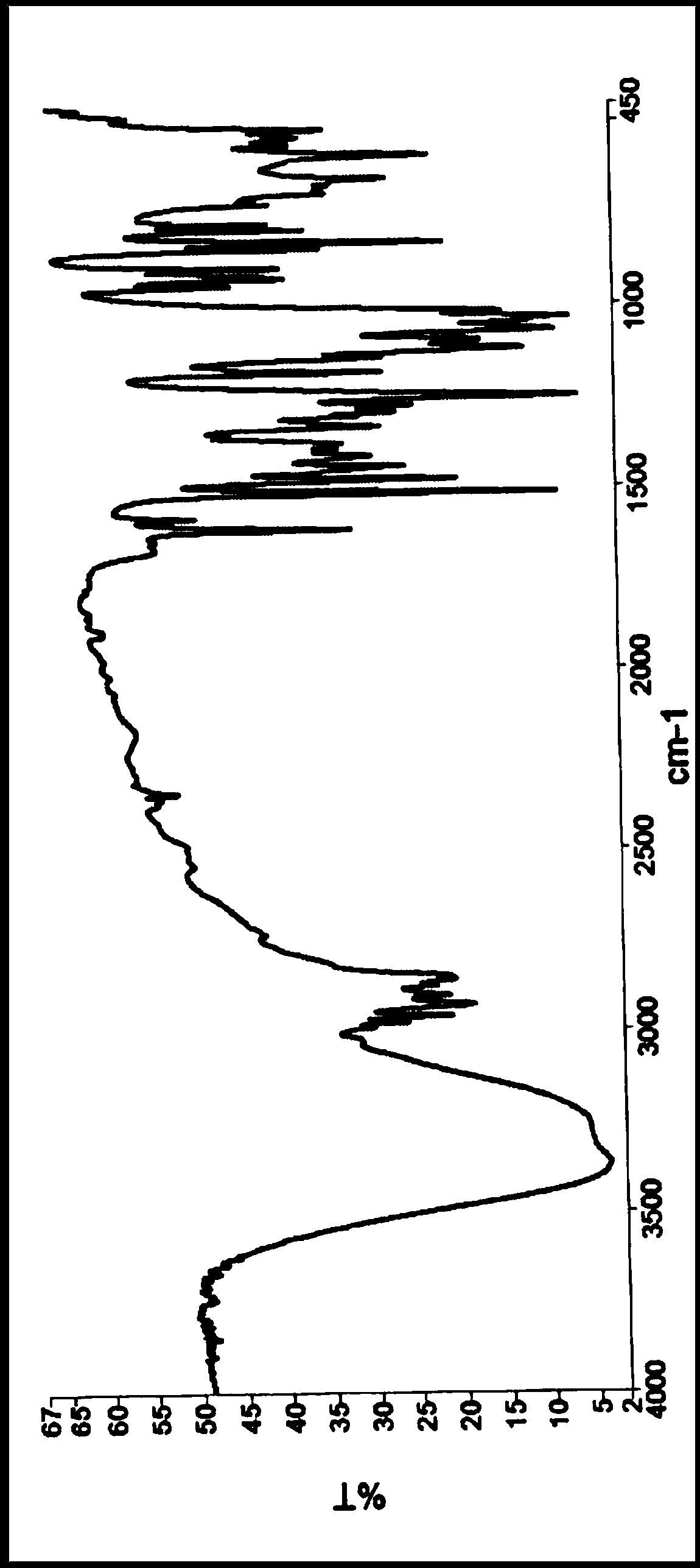
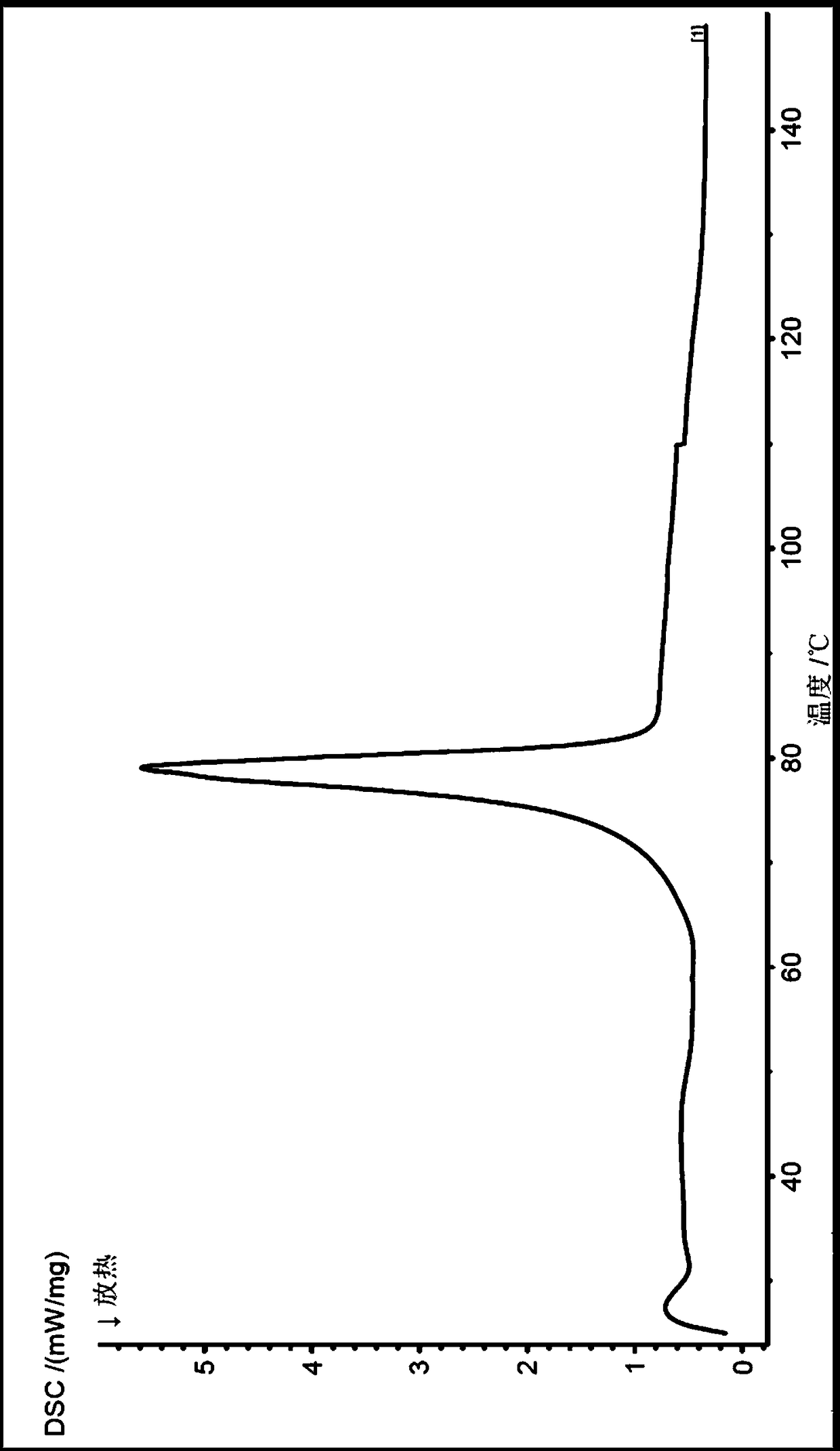
The invention discloses an isavuconazole monohydrate crystal form and further discloses a preparation method thereof. The method comprises the following steps: by taking (2S,3R)-3-(2,5-difluoro)-3-hydroxyl-2-methyl-4-(1H-1,2,4-triazole-1-yl) butyronitrile, diethylphosphorodithioate and 2-bromo-4 cyan-acetophenone as raw materials, carrying out steps of an addition reaction and penta-heterocycle closing and a unique post-treatment and crystallization process to prepare isavuconazole monohydrate which is white crystalized solid powder. The solid powder has the crystal form when being detected by XRPD (X-ray Powder Diffraction). Compared with previous isavuconazole amorphous powder, isavuconazole monohydrate crystal powder prepared by the process has the advantages of simple crystallization process, high operability, good stability and the like, and industrialized production is easier to realize.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Method for separating out nafcillin acid crystal from nafcillin sodium water solution
The invention relates to a method for separating out nafcillin acid crystal from a nafcillin sodium aqueous solution, which comprises the following steps: adding one of ethyl acetate, butyl acetate, n-butanol, n-hexane or petroleum ether as an extracting agent into the nafcillin sodium aqueous solution, and dripping an acidifying agent into the solution to make the pH value to be between 1.5 and 2.0 so as to separate out the crystal; and separating out the crystal, washing the crystal by purified water to make the pH value of washing liquor to be 4.0, drying the crystal at a temperature of between 45 and 55 DEG C to ensure that the moisture is below 3 percent to obtain the nafcillin acid crystal. The nafcillin acid crystal separated out by the extracting agent has the advantages of good crystal form, easy filtration, easy drying, less residual solvent in a product, and good quality; and the content of nafcillin acid is more than 93 percent. The crystallization method has the advantages of simple crystallize process, single extracting agent, and easy reclamation.
Owner:朗致集团江西医药有限公司
Midostaurin new crystal forms and preparation method and application thereof
PendingCN112812129ASimple crystallization processHigh purityOrganic active ingredientsOrganic chemistry methodsMidostaurinChemical stability
The invention relates to midostaurin novel crystal forms A, B and C as well as a preparation method and application of the novel crystal forms A, B and C. The crystal forms have excellent properties in the aspects of physical and chemical stability and processing adaptability. The crystallization process is simple and convenient to operate, and industrial production can be realized.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Crystal forms of clorsulon as well as preparation methods and applications of crystal forms
InactiveCN107868024AImprove solubilitySimple crystallization processOrganic active ingredientsOrganic compound preparationChemical stabilityPhysical chemical
The invention relates to novel crystal forms A, B, C and D of clorsulon as well as preparation methods and applications of the crystal forms. The crystal forms have excellent properties in physical and chemical stability and processing adaptability.
Owner:ZHEJIANG HISUN PHARMA CO LTD
A novel industrial crystallization method of cefathiamidine and its preparation
ActiveCN104926833BPromote enrichmentSimple crystallization processOrganic chemistry methodsBulk chemical productionSolventImpurity
The invention discloses a novel industrial crystallizing technology for cefathiamidine, Recrystallization of the cefathiamidine is achieved by adopting the mode of combining the supercritical fluid extraction technology and the traditional crystallizing technology. In the whole crystallizing system, the process of extraction, adsorption, crystallization and drying is completed under the joint effect of supercritical fluid, a solvent, an extraction pool and a crystallizing pool under the condition of specific temperature and pressure, and recrystallization of the cefathiamidine is achieved. The method is high in separation efficiency and product purity and few in impurities, and greatly improves the quality of preparation products.
Owner:HAINAN LINGKANG PHARMA CO LTD +1
New penicillin crystal preparation and preparation method thereof
ActiveCN104130270BSmall particlesUniform particle size distributionAntibacterial agentsOrganic chemistry methodsSide effectFiltration
The invention discloses an azlocillin sodium crystal preparation and a preparation method thereof. The preparation method comprises the following steps: step 1, dissolving a crude azlocillin sodium product in a mixed solvent of methanol and ethyl methyl ketone, adding active carbon for adsorption, carrying out filtering, cooling the obtained filtrate to allow a crystal to be precipitated, washing the crystal with a mixed solution of methanol and ethyl methyl ketone and carrying out pressure-reduced drying so as to obtain a primary refined azlocillin sodium product; and step 2, dissolving the primary refined azlocillin sodium product in a hot ethyl methyl ketone solution, carrying out press filtration with a high-efficiency filter, subjecting obtained mixed liquor to cooling, pressure reduction and concentration, then carrying out cooling to allow a crystal to be precipitated and subjecting the precipitated crystal to filtering and pressure-reduced drying so as to obtain the azlocillin sodium crystal preparation. The azlocillin sodium crystal preparation provided by the invention has the advantages of a small particle size, uniform particle size distribution, good fluidity, simple crystallization process, low residual quantity of the organic solvent, high stability, good security, small toxic and side effects, low proneness to allergy, etc.
Owner:BAIDE LIXI HEALTH CARE
Novel crystal form of EGFR inhibitor, and preparation method thereof
PendingCN113045541ALow toxicityImprove stabilityOrganic chemistry methodsCombinatorial chemistryCrystallinity
The invention provides a novel crystal form of an EGFR inhibitor, and a preparation method thereof, and belongs to the field of medicinal chemistry. The crystal form has the characteristics of good stability and low toxicity. The preparation method comprises the following steps: suspending and stirring the EGFR inhibitor and a solvent, filtering, drying, and placing the dried product in humid air to obtain the product. The preparation method is simple to operate, mild in condition, good in crystallinity and easy for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
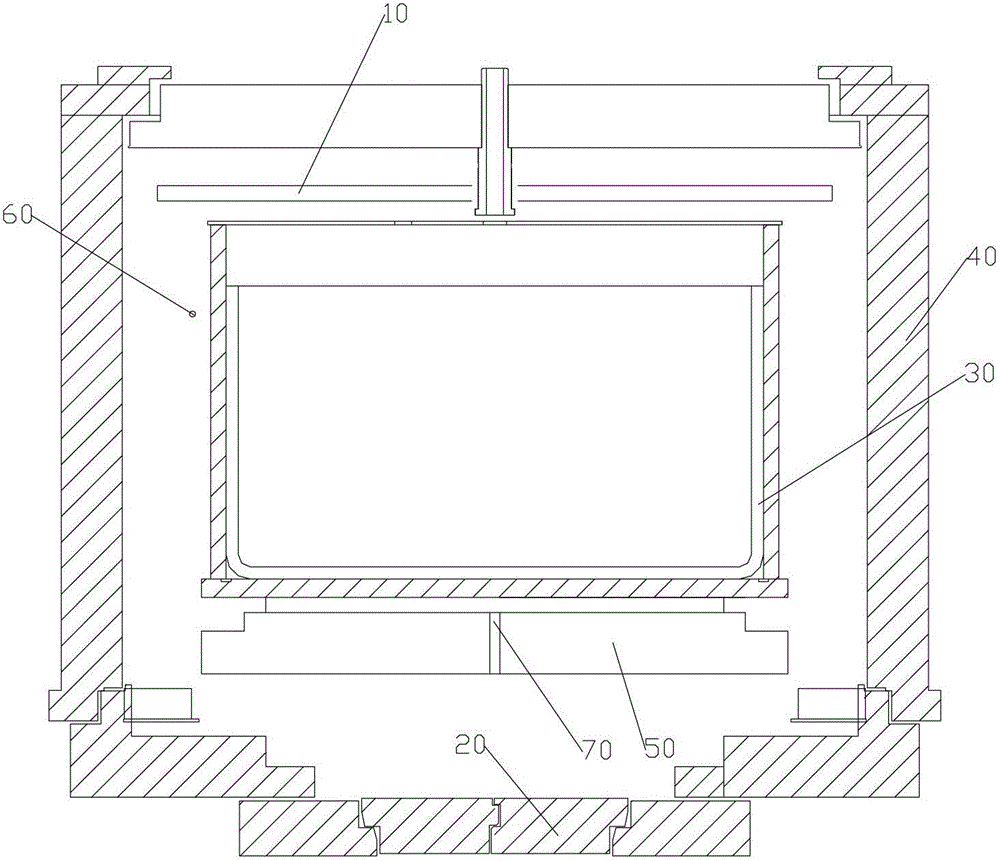
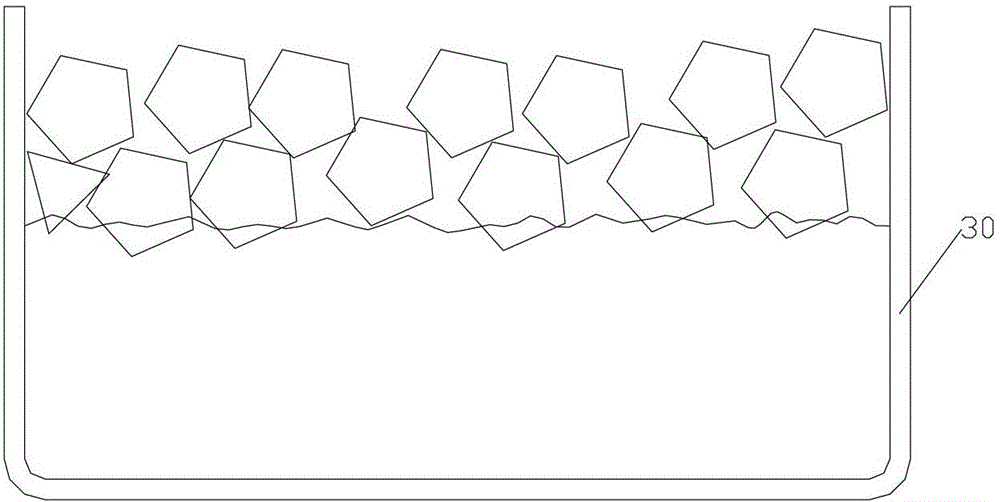
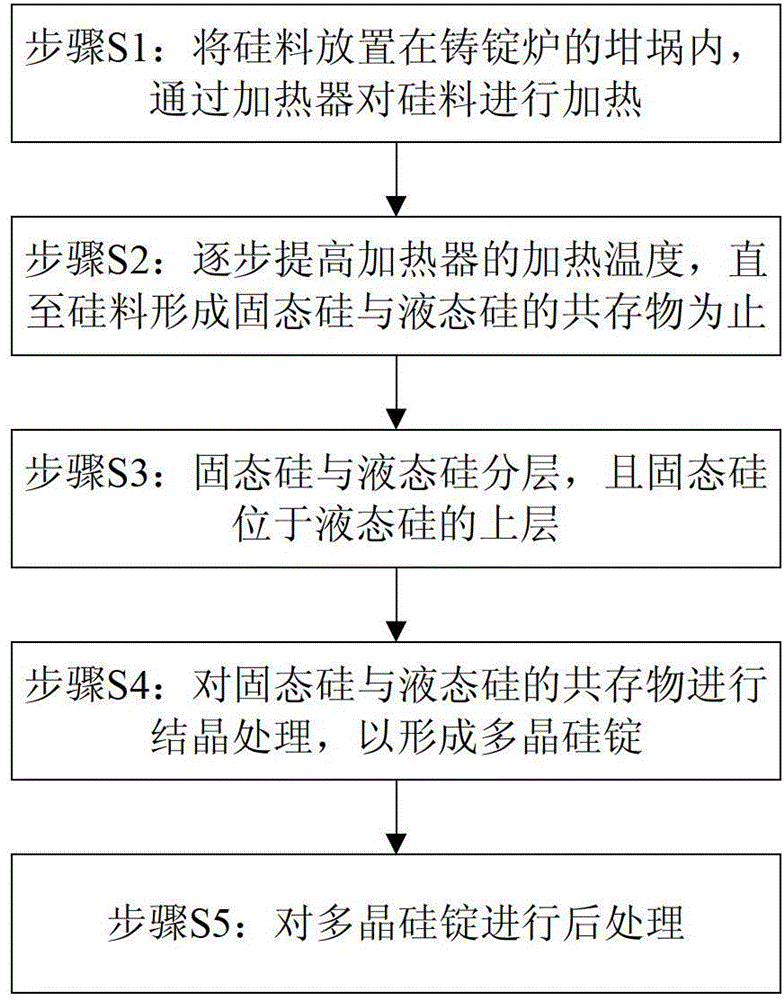
Crystallization process of polycrystalline silicon and ingot casting process of polycrystalline silicon
ActiveCN103352248BConsistent temperatureConsistent latent heatPolycrystalline material growthSingle crystal growth detailsIngot castingMicrocrystalline silicon
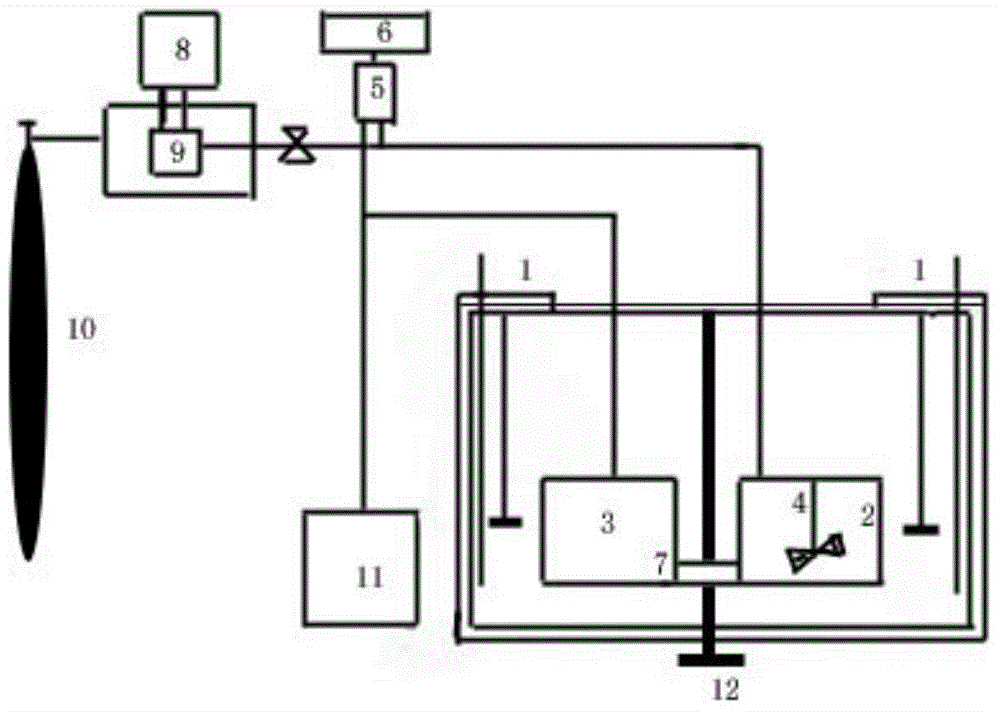
The invention provides a crystallization process of polycrystalline silicon and an ingot casting process of polycrystalline silicon. The crystallization process comprises the following steps: when a silicon material is in the state of coexistence of solid silicon and liquid silicon, the temperature of the top of an ingot furnace is kept so as to continuously melt solid silicon, and the temperature of the top in the ingot furnace is gradually lowered to enable the liquid silicon to be subjected to recrystallization to form the polycrystalline silicon ingot. The crystallization treatment is performed to the liquid silicon, when the silicon material is in the state of mixing of solid and liquid, so the temperatures of liquid silicon in all the ingot furnaces are basically the same, latent heat of the liquid silicon is the same, and therefore the crystallization velocity of the liquid silicon is enabled to be stable, the crystallization stability of liquid silicon is improved, and the properties of solar cells manufactured by the polycrystalline silicon ingot is improved. Meanwhile, the crystallization process of the polycrystalline silicon has the characteristics of simple process and high production efficiency.
Owner:YINGLI ENERGY CHINA
Crystallization process and application of preparing inositol with high crystallinity and large particle size
ActiveCN105669376BAvoid it happening againAvoid chippingCosmetic preparationsHydroxy compound active ingredientsPolymer scienceFiltration
The invention belongs to the technical field of medicament crystallization processes, and discloses a crystallization process for preparing high-crystallinity myo-inositol with a large particle size. A product prepared by the crystallization process is columnar crystalline myo-inositol, a particle size thereof can be more than 100 microns, the product is high in crystallinity and uniform in particle size distribution, and the shortcomings of low content, high moisture absorption and caking rate, long suction filtration and drying time, poor flowability and the like of powdery myo-inositol prepared by the prior art are overcome. The crystallization process has the characteristics of process simplicity, low equipment requirements and the like, production of a dihydrate crystal form H in a process is avoided, caking and crystal breaking in a drying process are avoided, suction filtration and drying time is shortened, and process cost is reduced.
Owner:ZHUCHENG HAOTIAN PHARMA
Tedirosin 1,4-dioxane solvent compound and preparation method
ActiveCN106008629BUniform particle size distributionPrevent coalescenceSugar derivativesOrganic chemistry methodsSolventEnergy consumption
The invention provides a tildipirosin 1,4-dioxane solvate and a preparation method thereof. Characteristic peaks occur in an X-ray powder diffraction pattern represented by 2theta when 2theta is equal to 6.06+ / -0.2, 8.42+ / -0.2, 9.34+ / -0.2, 9.94+ / -0.2, 10.28+ / -0.2, 12.34+ / -0.2, 13.44+ / -0.2, 13.74+ / -0.2, 14.66+ / -0.2, 15.58+ / -0.2, 15.78+ / -0.2, 16.40+ / -0.2, 17.00+ / -0.2, 17.90+ / -0.2, 18.60+ / -0.2, 19.32+ / -0.2, 20.10+ / -0.2, 21.40+ / -0.2, 22.34+ / -0.2, 22.68+ / -0.2, 23.34+ / -0.2 and 24.18+ / -0.2. The preparation method employs a constant-temperature suspended crystal transformation process and is simple in process and low in energy consumption. The tildipirosin 1,4-dioxane solvate is in a rod shape and crystal is of a regular shape.
Owner:TIANJIN UNIV
A kind of preparation method of sofosbuvir crystal form 6
InactiveCN104829673BAchieve purificationAchieve refinementSugar derivativesSugar derivatives preparationSolventCrystallization
The invention discloses a novel crystallization method for preparing a sofosbuvir crystal form 6. Compared with an original preparation method of the crystal form 6, the novel crystallization method disclosed by the invention has the advantages that the purification effect is good, products are easy to dry, an environment-friendly effect is achieved and the like since sofosbuvir is prepared from a solution containing various organic mixed solvents through cooling crystallization.
Owner:CHARM PHARMATECH NANJING
A kind of sodium acetate production process
ActiveCN109734582BHigh purityDiffusion fastSolution crystallizationCarboxylic acid salt preparationSodium acetateSodium acetrizoate
The invention relates to a sodium acetate production technology. The sodium acetate production technology comprises the following steps: synthesizing, purifying, filtering, concentrating, crystallizing, centrifuging and spin-drying, checking, and packaging. A crystallizing device of the sodium acetate production technology is different from a current jacket-type indirect contact cooling device. Acold gas in a lower temperature is used to be fed into concentrated liquid for cooling, diffusion of the cold gas and the liquid is rapid, and contact is uniform, so cooling efficiency is better. Through installing multiple cold gas pipelines, a heater and an air pressure sensor, a problem of blocking and coagulating an air outlet can be rapidly detected and solved, so a crystallizing flow is simpler, and easy to automatically operate and control. An obtained sodium acetate trihydrate product is higher in purity, and reaches a detecting requirement.
Owner:台山市新宁制药有限公司
Polycrystalline silicon crystallization process
InactiveCN106757335AConsistent temperatureConsistent latent heatPolycrystalline material growthSingle crystal growth detailsRoom temperatureIngot
The invention relates to the technical field of polycrystalline silicon, and particularly discloses a polycrystalline silicon crystallization process which comprises the following steps: when a silicon mixture in an ingot furnace is in a state of coexistence of solid silicon and liquid silicon, maintaining the temperature of the inner top of the ingot furnace, after the solid silicon is completely molten, gradually reducing the temperature to room temperature at a speed of 5 to 10 DEG C per minute to recrystallize the liquid silicon to form a polycrystalline silicon ingot, crushing the polycrystalline silicon ingot into 60 meshes, and performing acid washing, cleaning and drying to obtain a polycrystalline silicon product. According to the crystallization process, crystallization treatment is performed ono the liquid silicon when the silicon mixture is in a solid-liquid mixture state, so that the temperature of the liquid silicon in each ingot furnace can keep basically consistent, the latent heat in the liquid silicon keeps consistent, the crystallization speed of the liquid silicon is stabilized, and the crystallization stability of the liquid silicon is improved. In addition, the polycrystalline silicon crystallization process has the characteristics of simple process and high production efficiency.
Owner:ANHUI ELECTRIC GRP SHARES
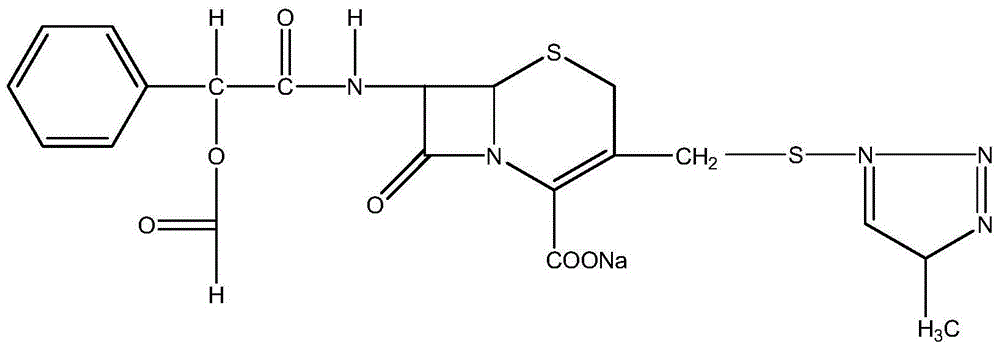
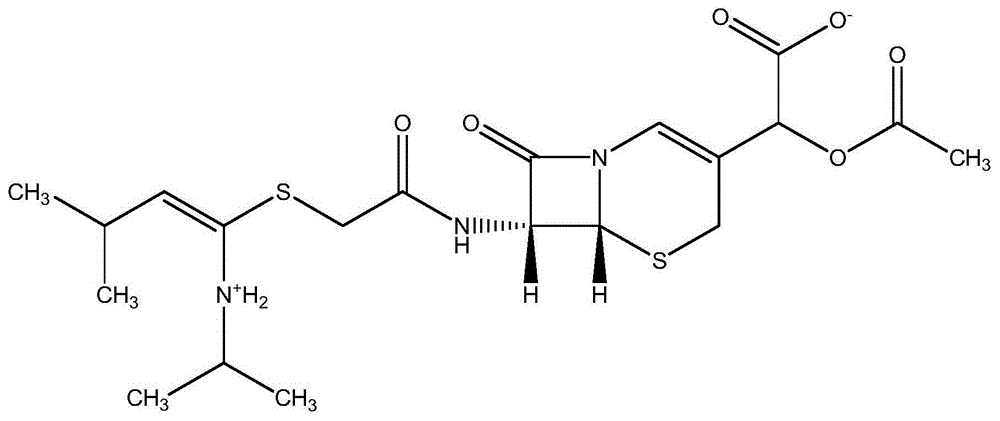
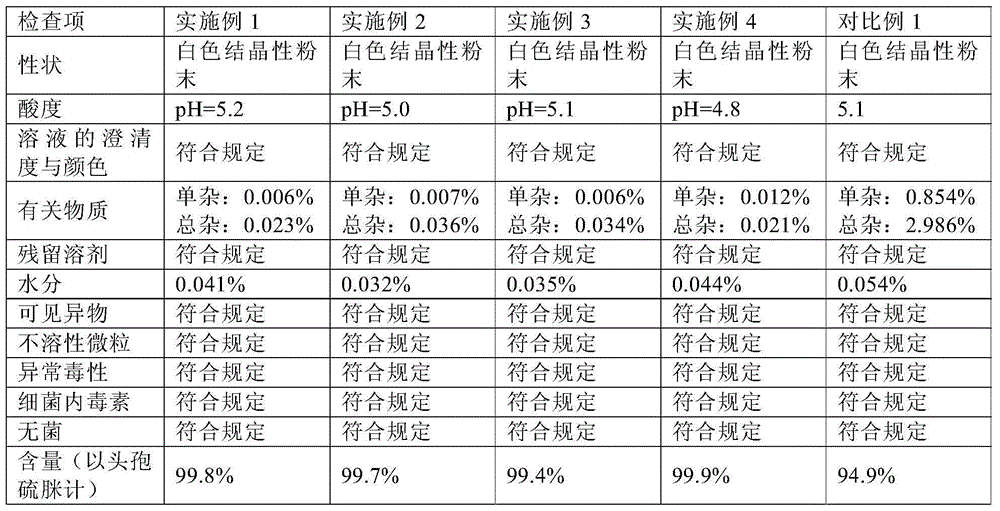
Cefodizime sodium compound solid, method for preparing same and pharmaceutical preparation of cefodizime sodium compound solid
ActiveCN102796118BSimple crystallization processUniform particle size distributionAntibacterial agentsOrganic active ingredientsActivated carbonAqueous acetone
The invention discloses a cefodizime sodium compound solid, a method for preparing the same and a pharmaceutical preparation of the cefodizime sodium compound solid, wherein the method for preparing the cefodizime sodium compound solid comprises the following steps of: first, dissolving crude cefodizime sodium salt in an acetone aqueous solution, then adding activated carbon to adsorb and filter, cooling the filtrate, stirring to separate the solid out, adding an acetone solvent to separate the solid out, filtering and drying, thus obtaining the cefodizime sodium compound solid. The prepared compound solids are fine particles, and have uniform particle size distribution and good liquidity, the crystallization technology is simple, the residual amount of organic solvent is low, and the product stability and pharmaceutical safety are effectively improved.
Owner:ZHEJIANG YATAI PHARMA
Tedizolid ammonium phosphate and its crystal form, preparation method and medical application
ActiveCN107226825BImprove solubilitySimple crystallization processAntibacterial agentsOrganic active ingredientsSolubilityPhosphate crystals
The invention relates to ammonium tedizolid phosphate, ammonium tedizolid phosphate crystal and their preparation method and medical use. The preparation method has simple processes and is conducive to industrial production. The ammonium tedizolid phosphate crystal A has good solubility. The preparation method has simple processes, is easy to operate, produces small pollution and can be industrialized. The ammonium tedizolid phosphate crystal drug has high purity, excellent physical and chemical properties, good chemical stability and reproducibility of processing. Compared with the tedizolid phosphate crystal, the ammonium tedizolid phosphate crystal A has good solubility, good clarity, a fast filtration rate, good hygroscopicity and good color.
Owner:ZHEJIANG HISUN PHARMA CO LTD
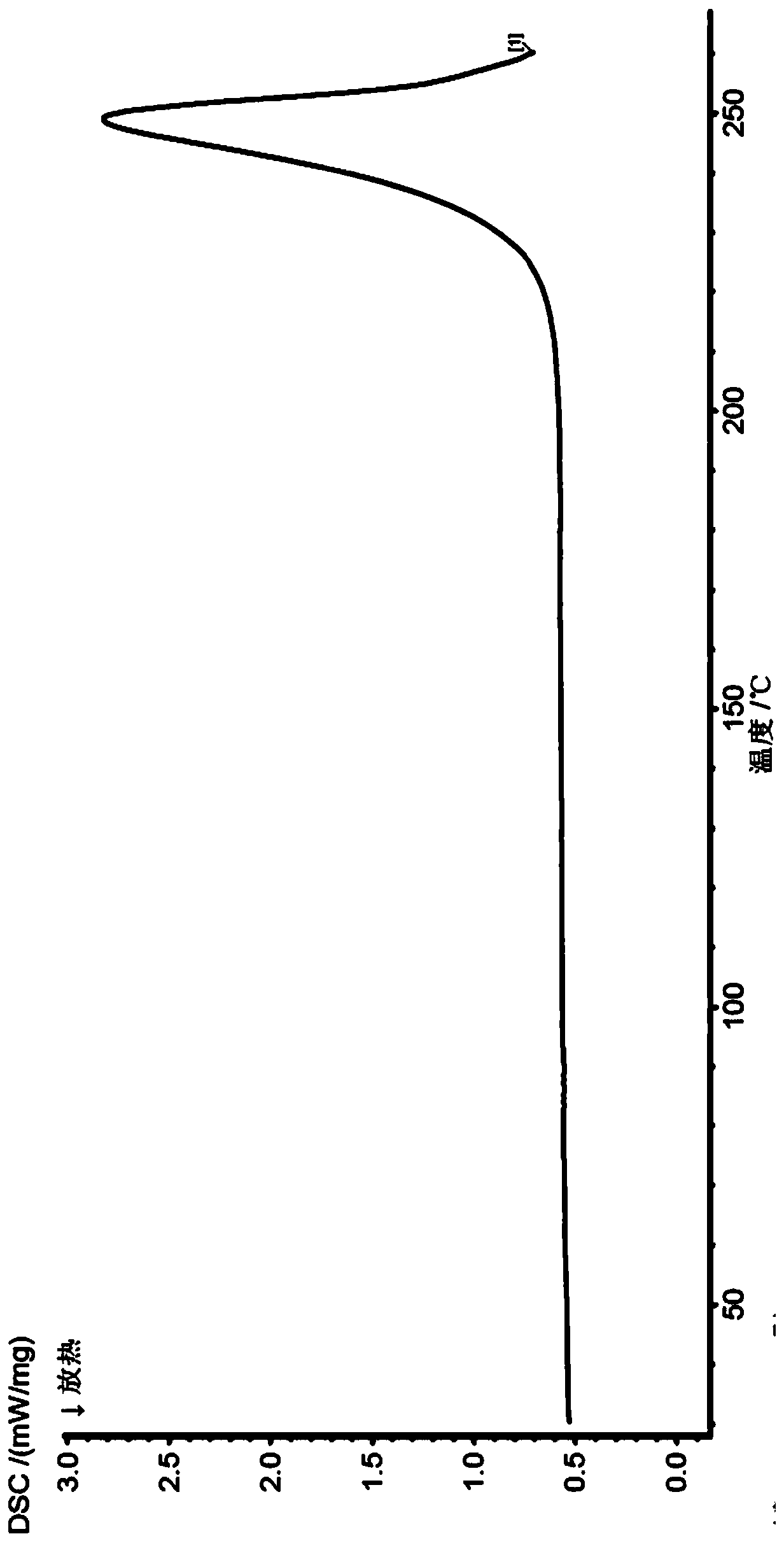
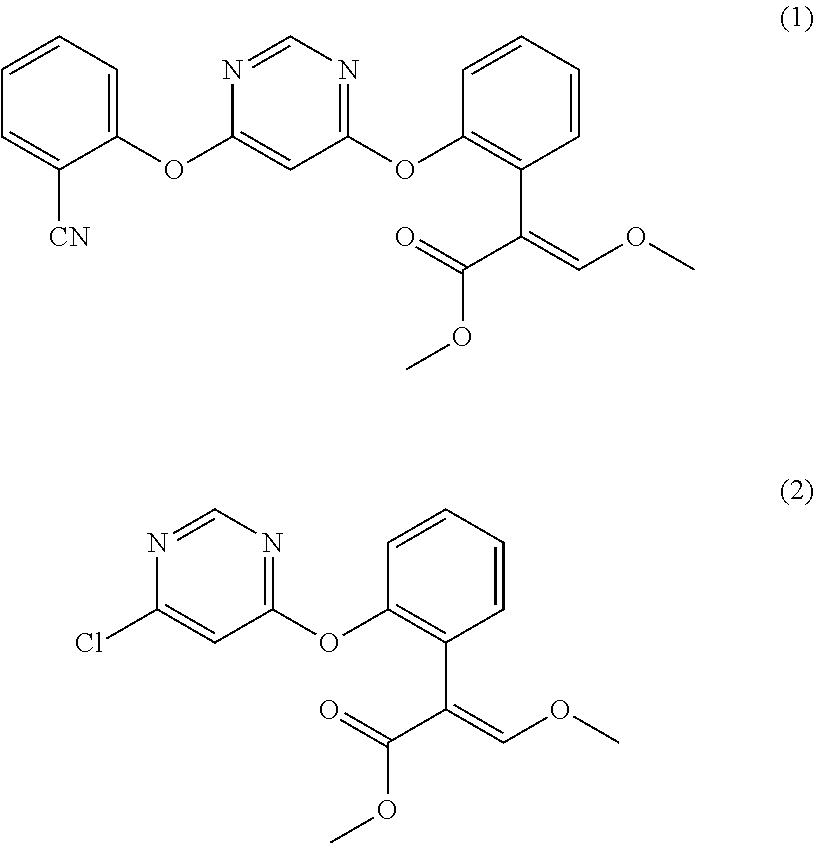
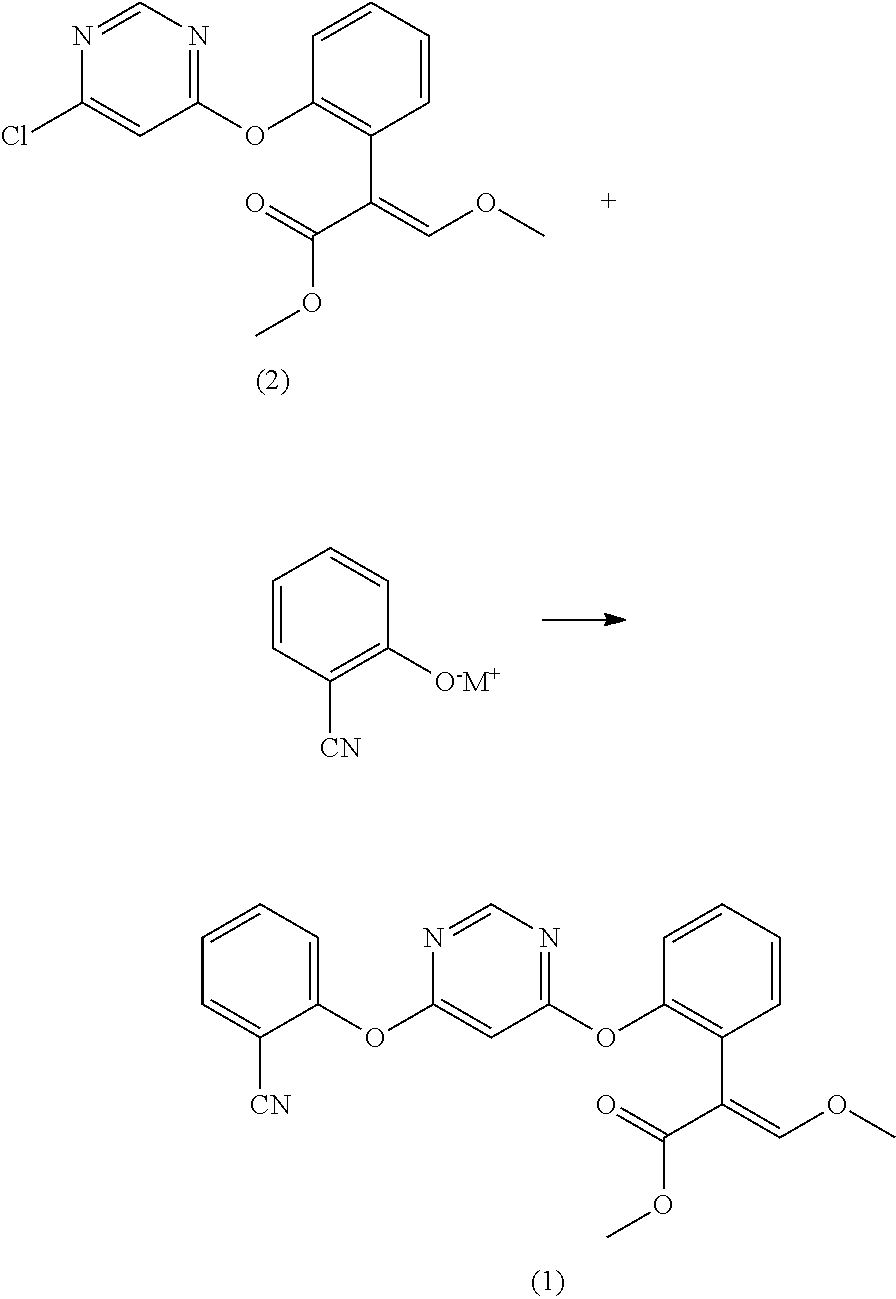
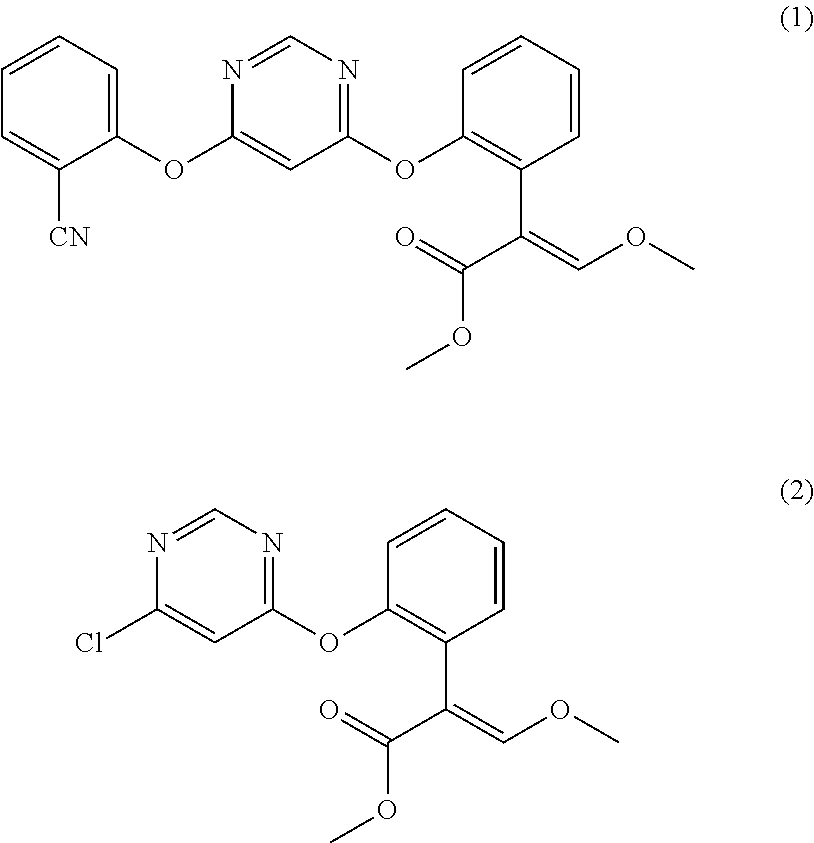
Preparation method for azoxystrobin
Disclosed in the present invention is a preparation method of azoxystrobin having a structure as shown by formula (1), the method comprising: a) performing an etherification reaction by reacting the compound having a structure shown by formula (2) with 2-cyanophenol and / or a salt thereof under the catalysis of an azabicyclo tertiary amine compound and / or a salt thereof as the catalyst in a butyl acetate medium to obtain a butyl acetate solution containing azoxystrobin; and b) cooling the butyl acetate solution containing azoxystrobin to precipitate Azoxystrobin having a structure as shown by formula (1) from the butyl acetate solution. Using the method provided by the present invention to prepare azoxystrobin can significantly improve the yield of azoxystrobin, and can obtain azoxystrobin products having high purity.
Owner:NUTRICHEM LAB CO LTD +1
A crystal form of pentostatin and its preparation method and application
ActiveCN108586556BImprove solubilityHigh purityOrganic active ingredientsSugar derivativesPharmaceutical SubstancesTableting
The present invention relates to a new crystal form I of pentostatin and its preparation method and use. The crystal form I has good solubility and is conducive to improving the intestinal absorption and oral bioavailability of the drug; the crystal form I of the present invention The medicine also has the advantages of high product purity, excellent physical and chemical properties, good chemical stability, and reproducible processing (filtration, drying, and tabletting); the crystallization process of the invention is simple, easy to operate, and less polluting, and can realize industrial production.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![2,2-dimethoxy-1,2-di[4-(METH)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition 2,2-dimethoxy-1,2-di[4-(METH)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition](https://images-eureka.patsnap.com/patent_img/c2cab4f9-24fb-48fe-9b3b-c08ae205af01/US20130324633A1-20131205-C00001.png)
![2,2-dimethoxy-1,2-di[4-(METH)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition 2,2-dimethoxy-1,2-di[4-(METH)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition](https://images-eureka.patsnap.com/patent_img/c2cab4f9-24fb-48fe-9b3b-c08ae205af01/US20130324633A1-20131205-C00002.png)
![2,2-dimethoxy-1,2-di[4-(METH)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition 2,2-dimethoxy-1,2-di[4-(METH)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition](https://images-eureka.patsnap.com/patent_img/c2cab4f9-24fb-48fe-9b3b-c08ae205af01/US20130324633A1-20131205-C00003.png)

![2,2-dimethoxy-1,2-DI[4-(meth)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition 2,2-dimethoxy-1,2-DI[4-(meth)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition](https://images-eureka.patsnap.com/patent_img/e22a4410-742c-40ce-a2bd-5f85845c2abf/US09068024-20150630-C00001.PNG)
![2,2-dimethoxy-1,2-DI[4-(meth)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition 2,2-dimethoxy-1,2-DI[4-(meth)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition](https://images-eureka.patsnap.com/patent_img/e22a4410-742c-40ce-a2bd-5f85845c2abf/US09068024-20150630-C00002.PNG)
![2,2-dimethoxy-1,2-DI[4-(meth)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition 2,2-dimethoxy-1,2-DI[4-(meth)acryloyloxy]phenylethane-1-one, method for producing the same, radical polymerization initiator and photocurable composition](https://images-eureka.patsnap.com/patent_img/e22a4410-742c-40ce-a2bd-5f85845c2abf/US09068024-20150630-C00003.PNG)