Novel crystal form of EGFR inhibitor, and preparation method thereof
A crystal form, 90%RH-95%RH technology, applied in the field of medicinal chemistry, can solve problems that have not yet been discovered, and achieve the effects of low toxicity, good repeatability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: Preparation of crystal form N1
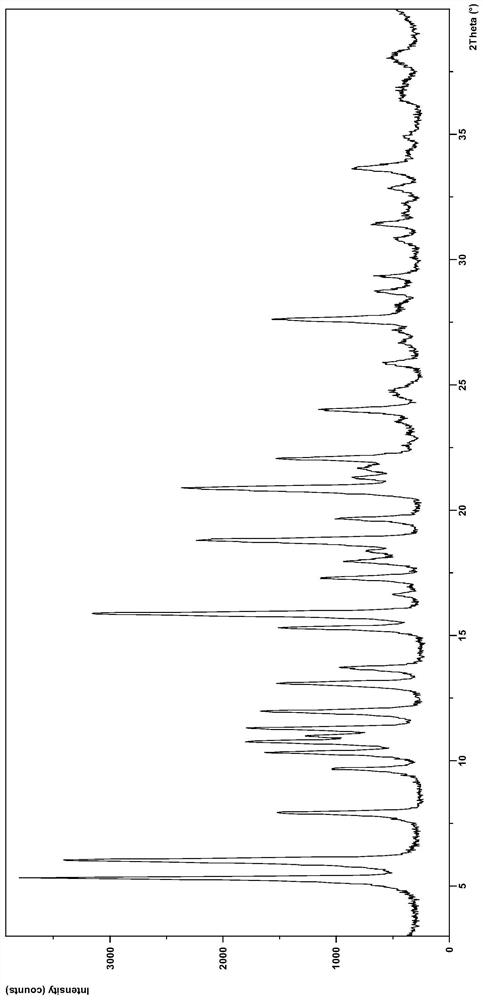
[0084] Weigh 200 mg of dacomitinib, suspend and stir in 10 mL of methanol at room temperature for 22 hours, filter and dry to obtain 162 mg of crystal form N1 product. After testing, its XRD pattern and figure 1 Basically the same.
Embodiment 2
[0085] Embodiment 2: the preparation of crystal form N2
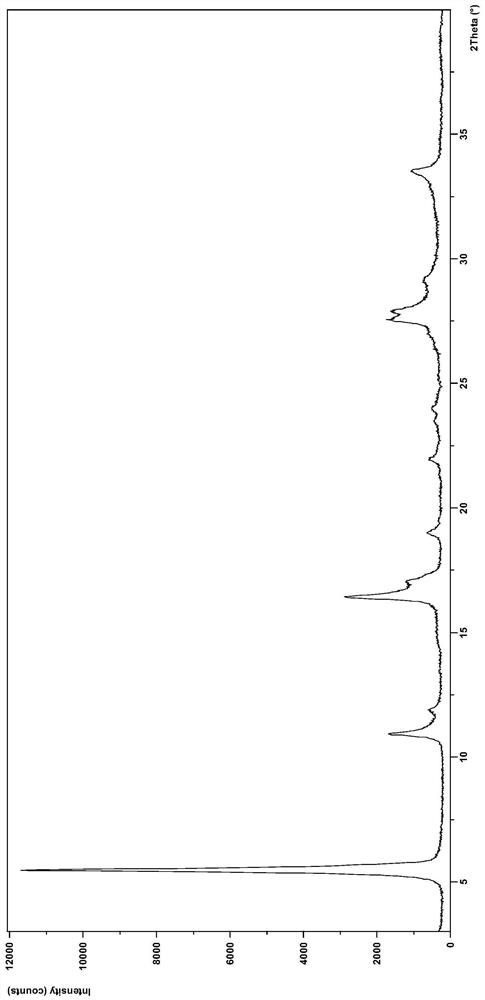
[0086] Weigh 300 mg of dacomitinib, dissolve it in 5 mL of tetrahydrofuran, and slowly add 10 mL of n-propanol dropwise to precipitate a solid, then filter and dry to obtain 240 mg of crystal form N2 product. After testing, its XRD pattern and figure 2 Basically the same.
Embodiment 3
[0087] Embodiment 3: Preparation of crystal form N3
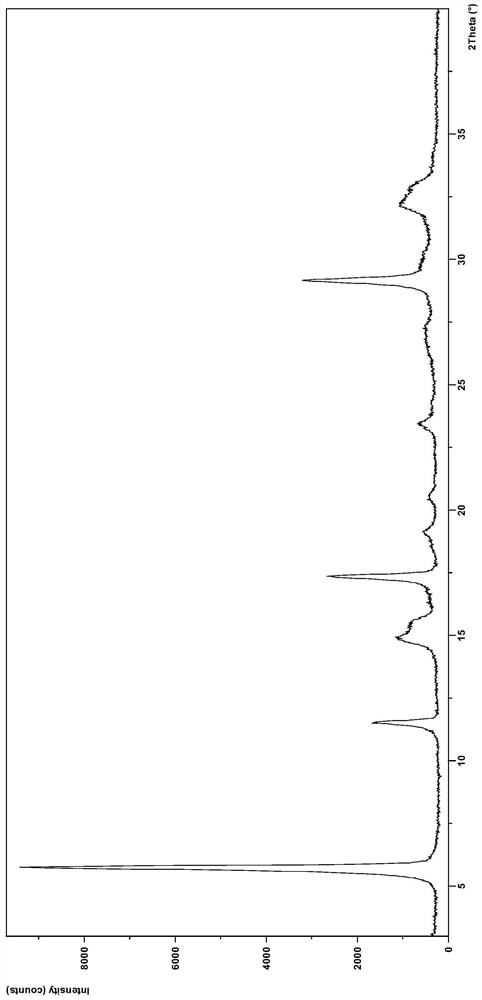
[0088] Weigh 200mg of dacomitinib, suspend and stir in 5mL of isopropanol at room temperature for 24h, filter and dry to obtain 156mg of crystal form N3 product. After testing, its XRD pattern and image 3 Basically the same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diffraction angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com