Crystallization process for preparing high-crystallinity myo-inositol with large particle size and application
A technology with high crystallinity and inositol, which is applied in the crystallization process and application field of inositol, can solve the problems of unavoidable agglomeration and crystal fragmentation, product residue cannot be removed, and the content of inositol is reduced, so as to avoid agglomeration and agglomeration Agglomerates and crystal fragmentation, avoiding agglomeration and crystal fragmentation, and improving high temperature and high humidity stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
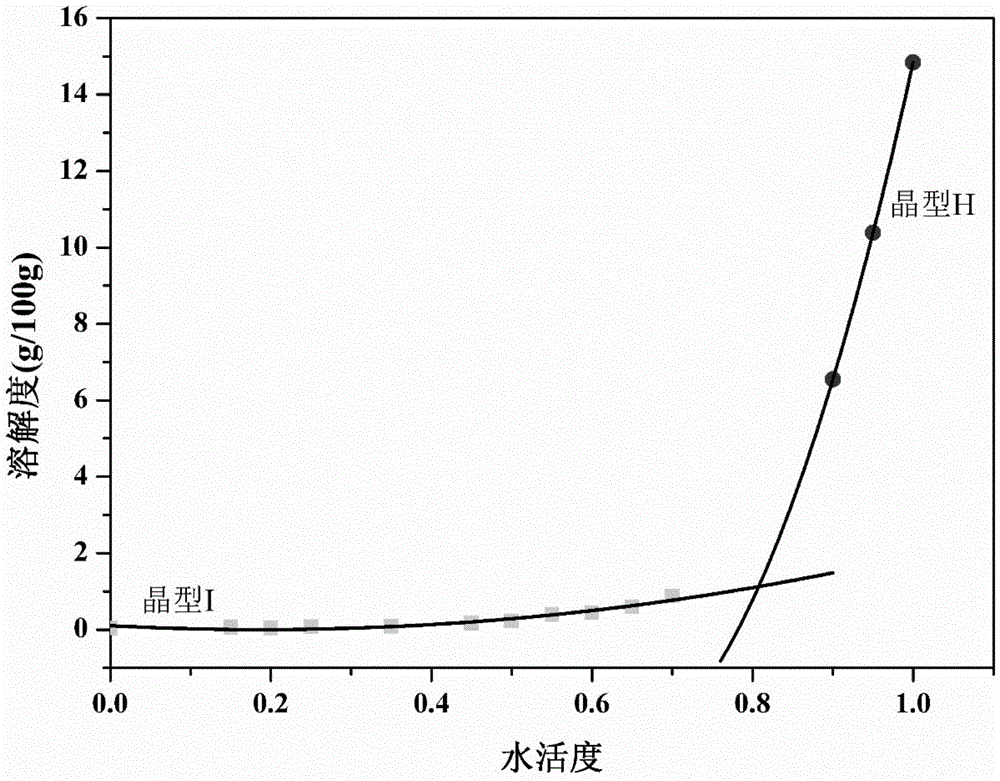
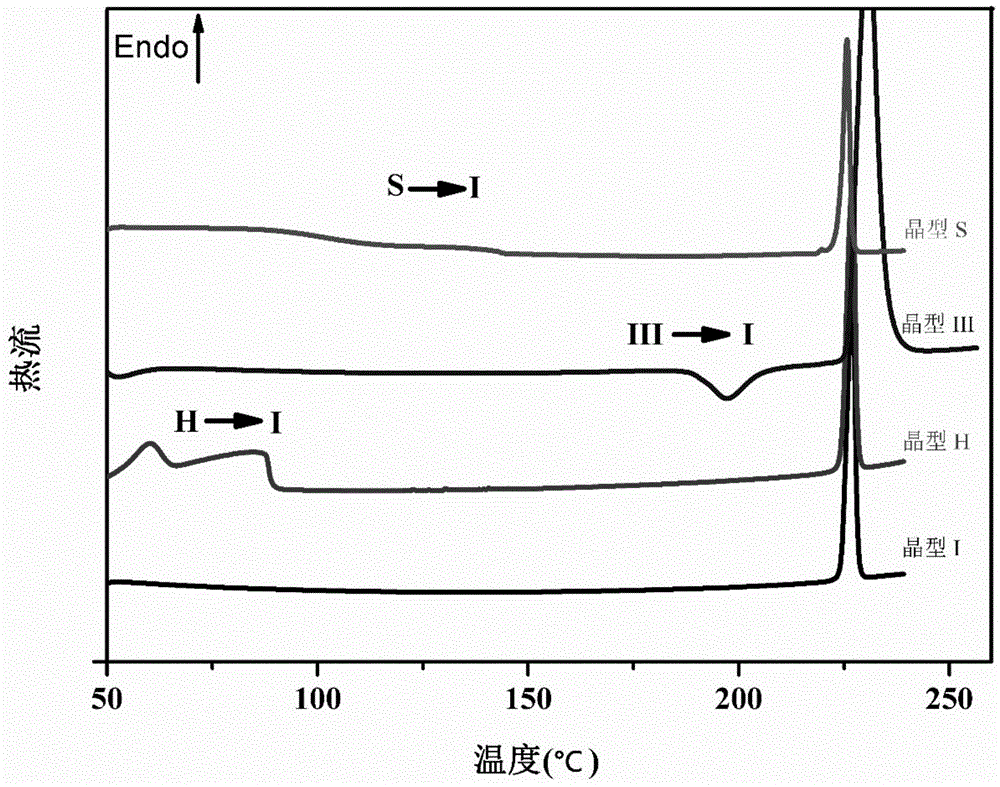
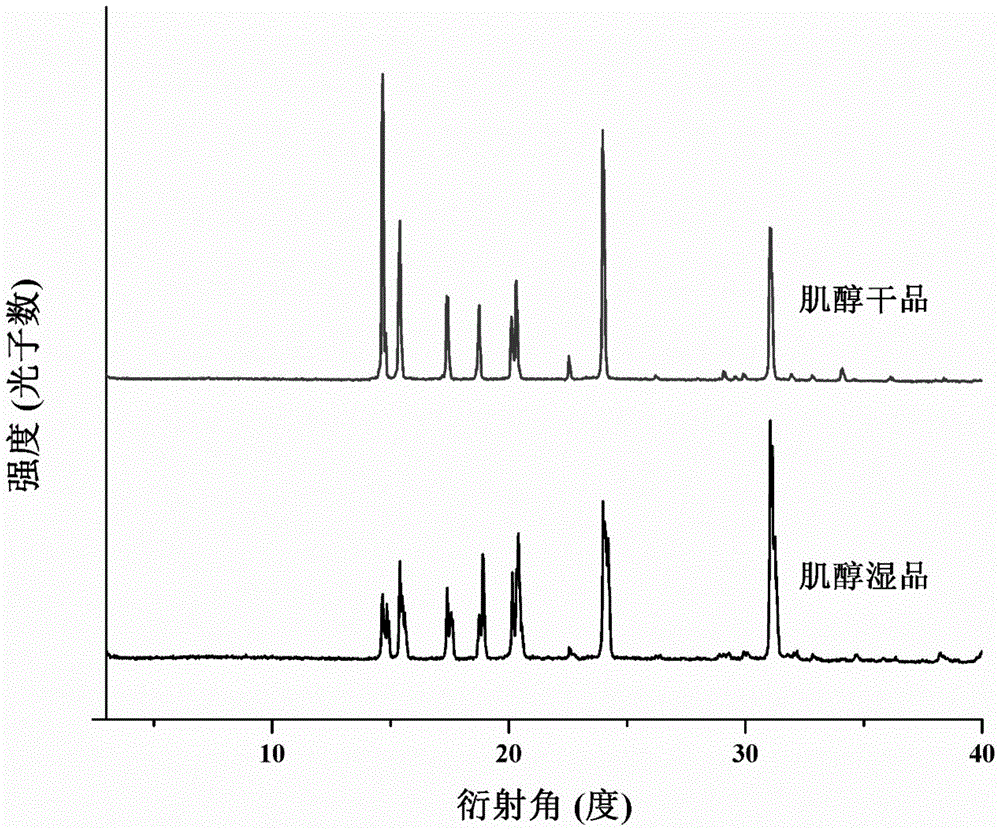
Image
Examples
Embodiment 1
[0034] Suspend 200g of fine inositol in 200g of ultrapure water, raise the temperature to 95°C, filter, add 10g of type I inositol seed crystals to the clear solution, keep the temperature for 1h, and then program the temperature drop at a rate of 3°C / h. When the temperature dropped to room temperature, it was filtered, and the wet product of inositol was dried in a blast drying oven at 50° C. to obtain 190 g of inositol crystals with a yield of 95%.
Embodiment 2
[0036] Suspend 200g of fine inositol in 300g of ultra-pure water, raise the temperature to 85°C, filter, add 10g of type I inositol seed crystals to the clear solution, keep the temperature for 1h, and then program the temperature down at a rate of 3°C / h. When the temperature dropped to room temperature, it was filtered, and the wet product of inositol was dried in a blast oven at 50° C. to obtain 176 g of inositol crystals with a yield of 88%.
Embodiment 3
[0038] Suspend 200g of fine inositol in 500g of ultra-pure water, raise the temperature to 65°C, filter, add 10g of type I inositol seed crystals to the clear solution, keep the temperature for 1h, and then program the temperature drop at a rate of 3°C / h. When the temperature dropped to room temperature, it was filtered, and the wet product of inositol was dried in a blast oven at 50° C. to obtain 164 g of inositol crystals with a yield of 82%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com