Crystal forms of clorsulon as well as preparation methods and applications of crystal forms
A cloxolone crystal form and cloxolone crystal technology are applied in the field of polymorph and the preparation of the crystal form, and can solve the problems of complicated preparation process, low crystal form purity, unfriendly environment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
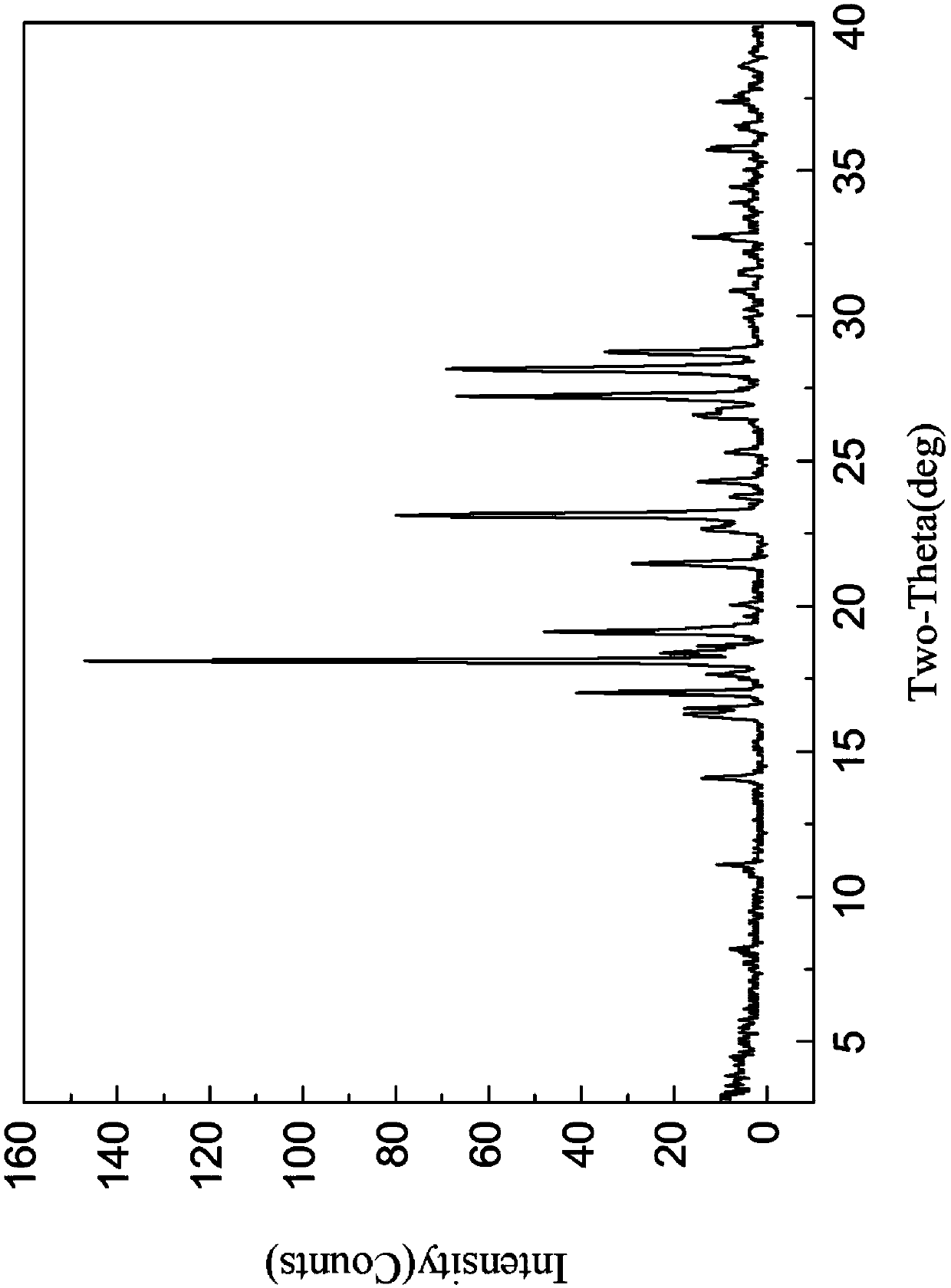
[0117] Add cloxolone 1g into water, wherein the weight volume ratio of cloxolone to water is 1:50; heat up to 90°C, and continue to stir for 60min to dissolve; add methyl isobutyl ketone, wherein, methyl isobutyl ketone The volume ratio of butyl ketone to water is 1:100; cool down to 25°C, crystallize; filter, vacuum dry at 45°C to obtain 0.5g crystals, HPLC=98.7%, X-ray powder diffraction pattern (XRD) , confirmed as Form A.
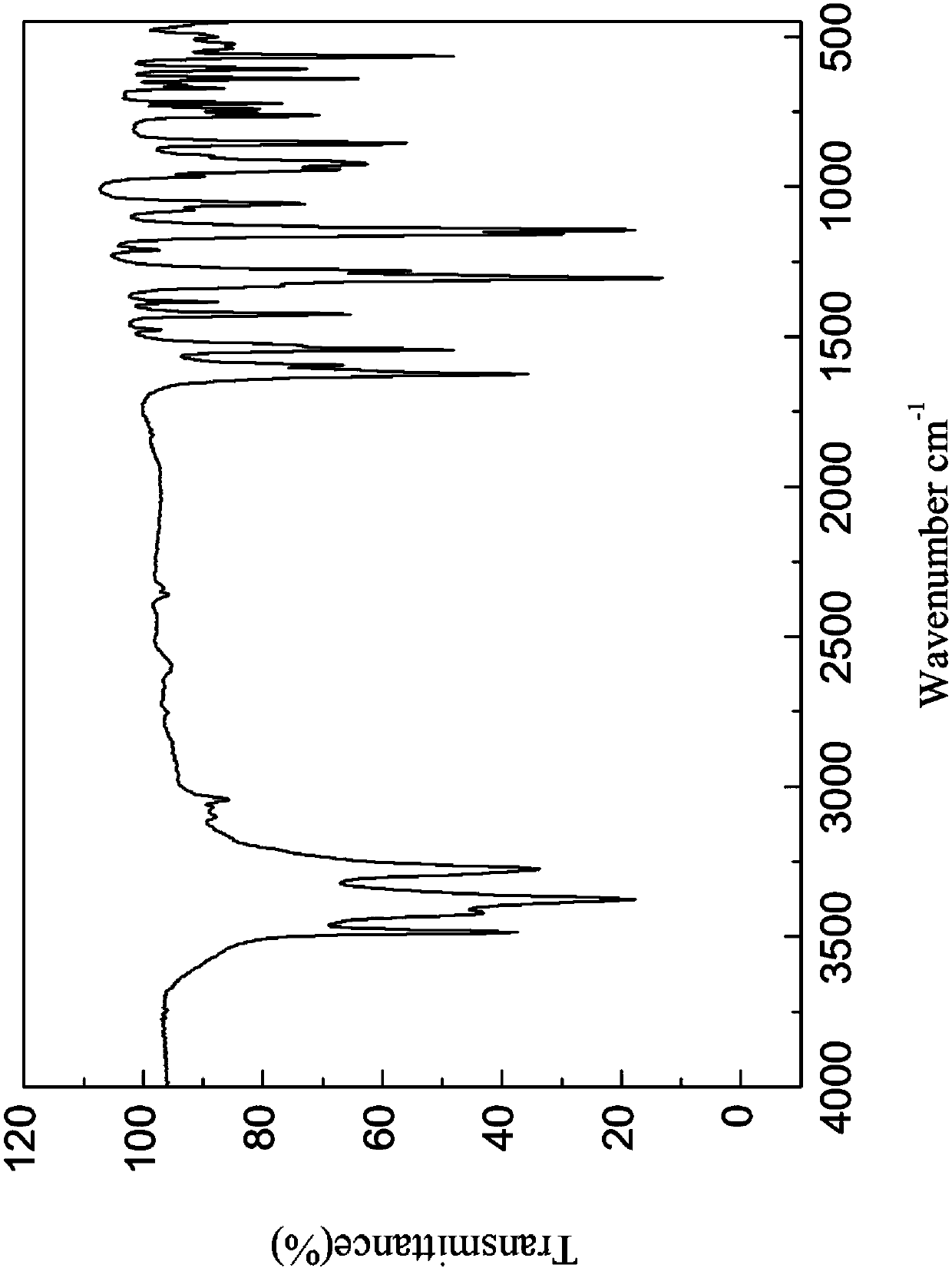
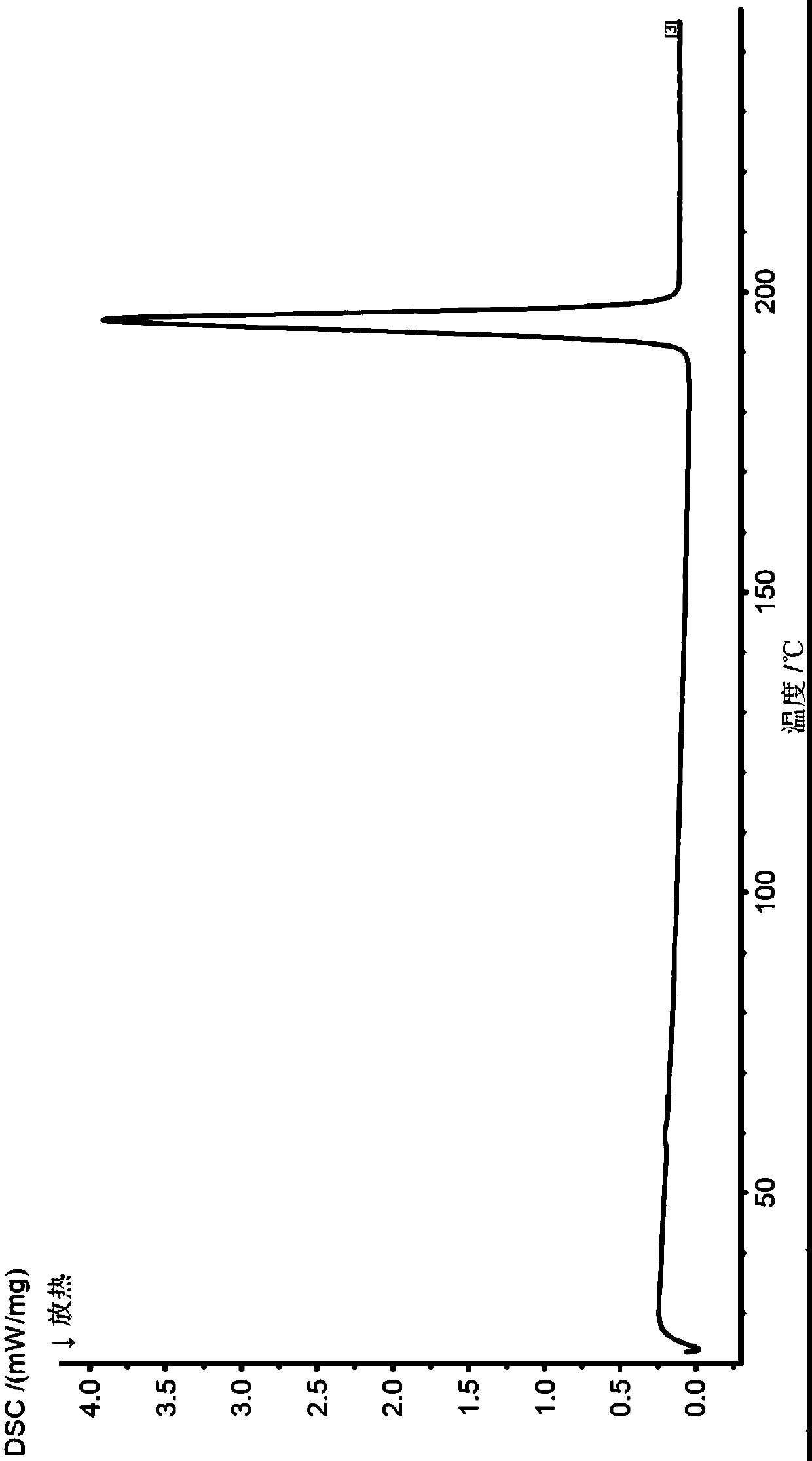
[0118] The X-ray powder diffraction, infrared, DSC and TGA spectra of this crystal form are detailed in Figure 1-4 , which is named Cloxolone Form A in the present invention.
Embodiment 2
[0120] Add cloxolone 1g into water, wherein the weight volume ratio of cloxolone to water is 1:50; heat up to 80°C, and continue to stir for 60min to dissolve; add methyl isobutyl ketone, wherein, methyl isobutyl ketone The volume ratio of butyl ketone to water is 1:100; cool down to 20°C, crystallize; filter, vacuum dry at 45°C to obtain 0.53g crystals, HPLC=98.6%, X-ray powder diffraction pattern (XRD) , confirmed as Form A.
Embodiment 3
[0122] Add cloxolone 1g into water, wherein the weight volume ratio of cloxolone to water is 1:50; heat up to 90°C, and continue to stir for 60min to dissolve; add methyl isobutyl ketone, wherein, methyl isobutyl ketone The volume ratio of butyl ketone to water is 1:100; cool down to 30°C, crystallize; filter, vacuum dry at 45°C to obtain 0.46g crystals, HPLC=98.8%, X-ray powder diffraction pattern (XRD) , confirmed as Form A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com