Preparation method of sofosbuvir crystal form 6
A technology of sofosbuvir crystals and sofosbuvir, which is applied in the field of medicine, can solve problems such as infeasibility, difficult process control, and lengthy steps, and achieve simple and efficient crystallization process, good repeatability, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] In the preparation method of crystal form 6 of the present invention, sofosbuvir all uses the sofosbuvir product (such as J.Med.Chem.2010,53,7202-7218) prepared by the method reported in existing literature; Other All solvents and reagents were commercially available chemically pure or analytically pure products.
[0043] The X-ray powder diffractometer used in the embodiment of the present invention is an X'pert PRO X-ray powder diffractometer from PANalytical Company. Cu-Kα rays are used, the test power is 40kV×250mA, the scanning speed is 5° / min, and the scanning range is 4-80° (2θ) θ-2θ continuous scanning. In the X-ray powder diffraction diagram obtained in the embodiment of the present invention, the horizontal axis represents the 2θ position of the diffraction peak (unit: degree); the vertical axis represents the intensity of the diffraction peak.
[0044]The differential scanning calorimetry-thermogravimetric (DSC-TGA) analysis instrument used in the embodiment...
Embodiment 1
[0047] Mix 1.0 g of sofosbuvir with 10.0 ml of cyclopentyl methyl ether and 1.0 ml of anisole, heat the mixture to 75±3°C and stir at 20 rpm to completely dissolve the solids, then cool within 90 minutes to 10±3°C and stand at this temperature for 48 hours, the precipitated white powdery crystals were taken out by filtration, washed with 3 ml of isopropyl ether and dried to obtain 0.92 g of product.
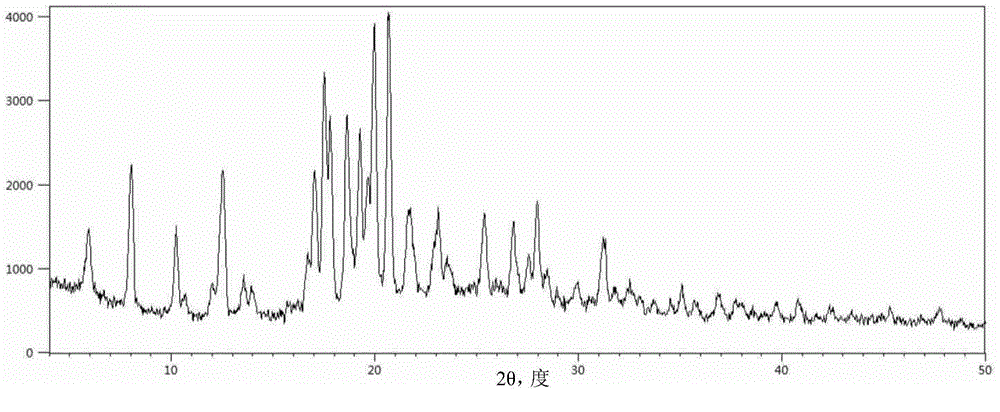
[0048] Carry out powder X-ray diffraction analysis to the obtained crystal sample, its result is as follows figure 1 shown. and figure 1 Correspondingly, the powder X-ray diffraction data of Sofosbuvir Form 6 are shown in Table 1.
[0049] The pXRD characteristic peak position of table 1 embodiment 1 product
[0050]
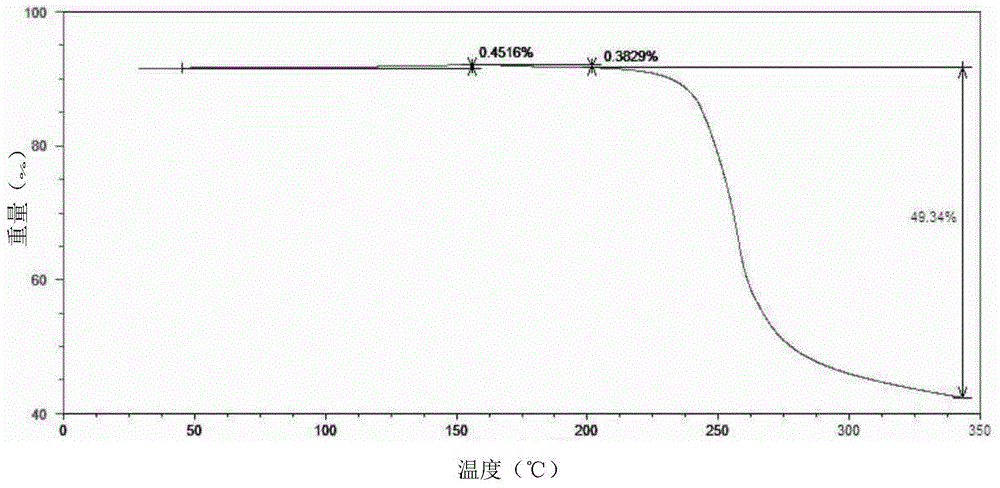
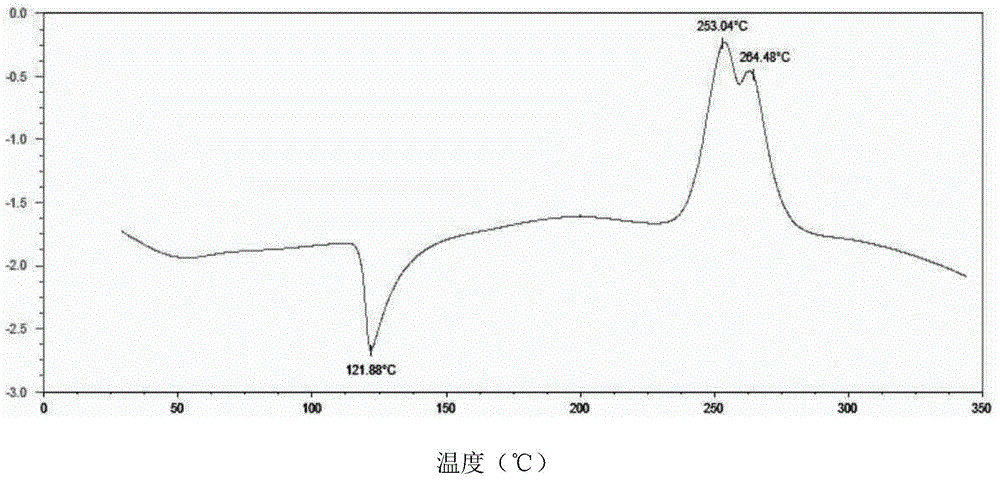
[0051] The powder sample was subjected to differential scanning calorimetry-thermogravimetric analysis, and the results were as follows: figure 2 shown. The shown pXRD, DSC-TGA and other test results are all the same as the characteristic spectrum of sofos...
Embodiment 2
[0053] Mix 1.0 g of sofosbuvir with 10.0 ml of cyclopentyl methyl ether and 2.0 ml of dibenzyl ether, heat the mixture to 75±3°C and stir at 20 rpm to completely dissolve the solid, then cool within 90 minutes to 5±3°C and stirred at this temperature for 48 hours, the precipitated white powdery crystals were taken out by filtration, washed with 3 ml of isopropyl ether and dried to obtain 0.93 g of the product with a yield of 93%. After the product was ground, pXRD, DSC-TGA and other tests were performed, all of which were identical to the characteristic spectrum of sofosbuvir crystal form 6 reported in the literature, confirming that the product was sofosbuvir crystal form 6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com