Method for preparing crystalline form 6 of Sofosbuvir
A non-buvir crystal form and crystallization technology, applied in the field of medicine, can solve the problems of unfeasible production, insufficient yield, large stirring resistance, etc., to avoid the use of flammable and explosive solvents, and the crystallization process. Simple and efficient, the effect of avoiding conversion steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
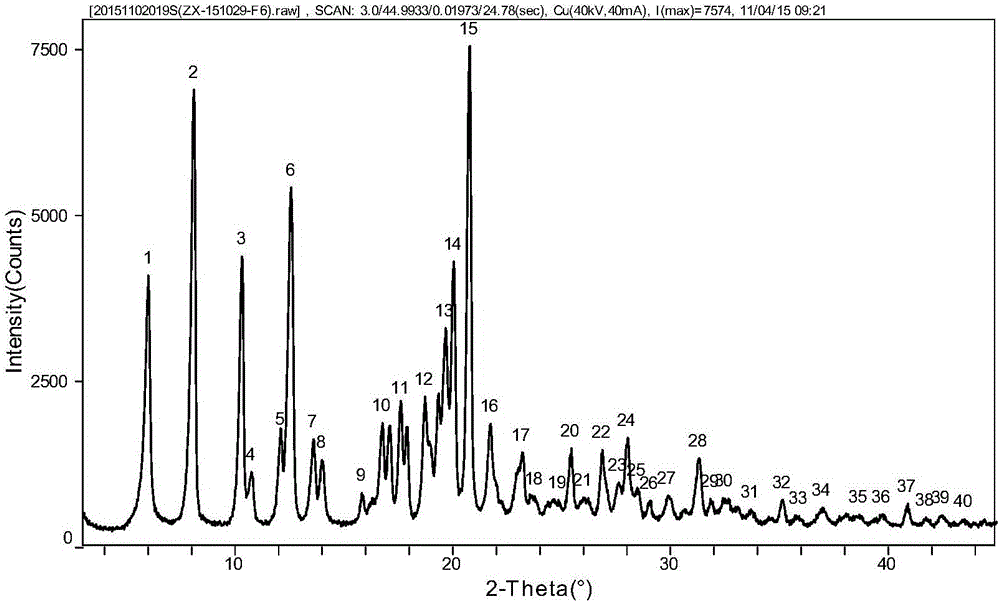
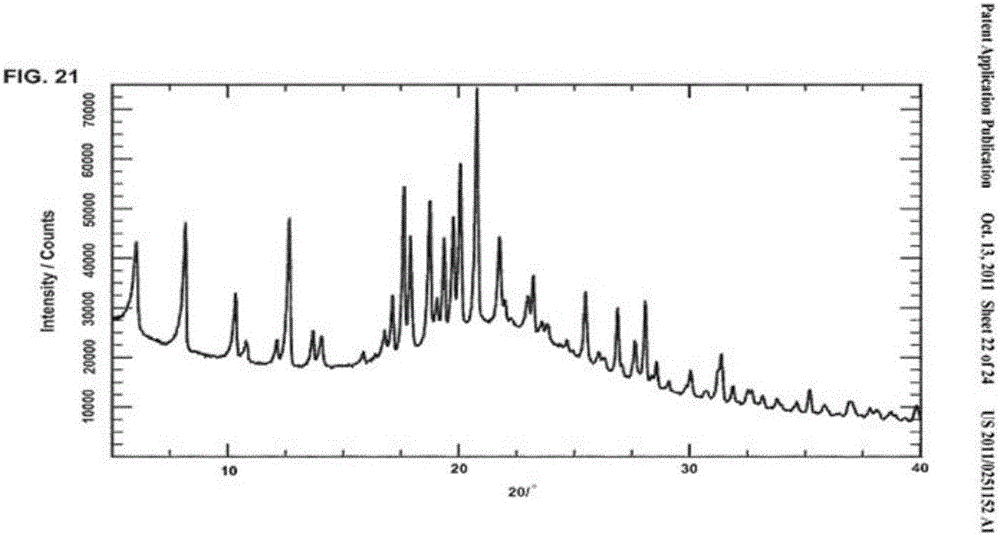
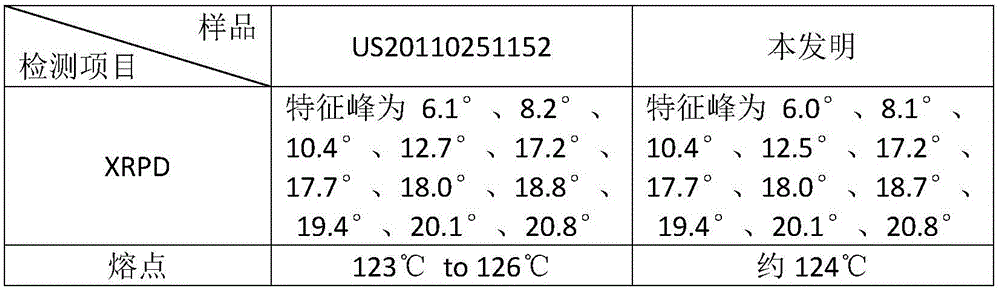
[0027] Mix 15g of sofosbuvir with 25g of methanol and 60g of water, heat to dissolve the solid completely; then cool to 20°C, and keep stirring for 2h; filter, and blow dry the filter cake at 55°C to obtain 13.5g of the product. Rate 90.0%. The XRPD pattern of the obtained sample is attached figure 1 shown.
Embodiment 2
[0029] Mix 15g of sofosbuvir with 20g of ethanol and 40g of water, heat to dissolve the solid completely, then cool to 15°C, keep stirring for 2h, filter, and dry the filter cake by blowing at 55°C to obtain 13.81g of the product. The rate is 92.07%.
Embodiment 3
[0031] Mix 15g of sofosbuvir with 25g of methanol and 80g of water, heat to dissolve the solid completely, then cool to 10°C, keep stirring for 4h, filter, and dry the filter cake by blowing at 55°C to obtain 14.2g of the product. The rate is 94.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com