Dapagliflozin crystal form and preparation method and purpose thereof
A crystal form, propylene glycol technology, applied in the field of chemical pharmaceuticals, can solve the problems of difficult preservation, storage, and transportation of solid API forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Dissolve 0.1g of Dapagliflozin (S)-propylene glycol hydrate (HPLC>99%) in 10ml of dichloromethane, raise the temperature to 32°C, and continue to stir for 30min to dissolve; filter, and magnetically stir to control the stirring speed to 300rpm, The filtrate was cooled to 18°C, stirred and crystallized at 18°C for 18h, filtered, and dried under vacuum (-0.1Mpa) at room temperature to obtain 0.056g crystals (HPLC>99%).
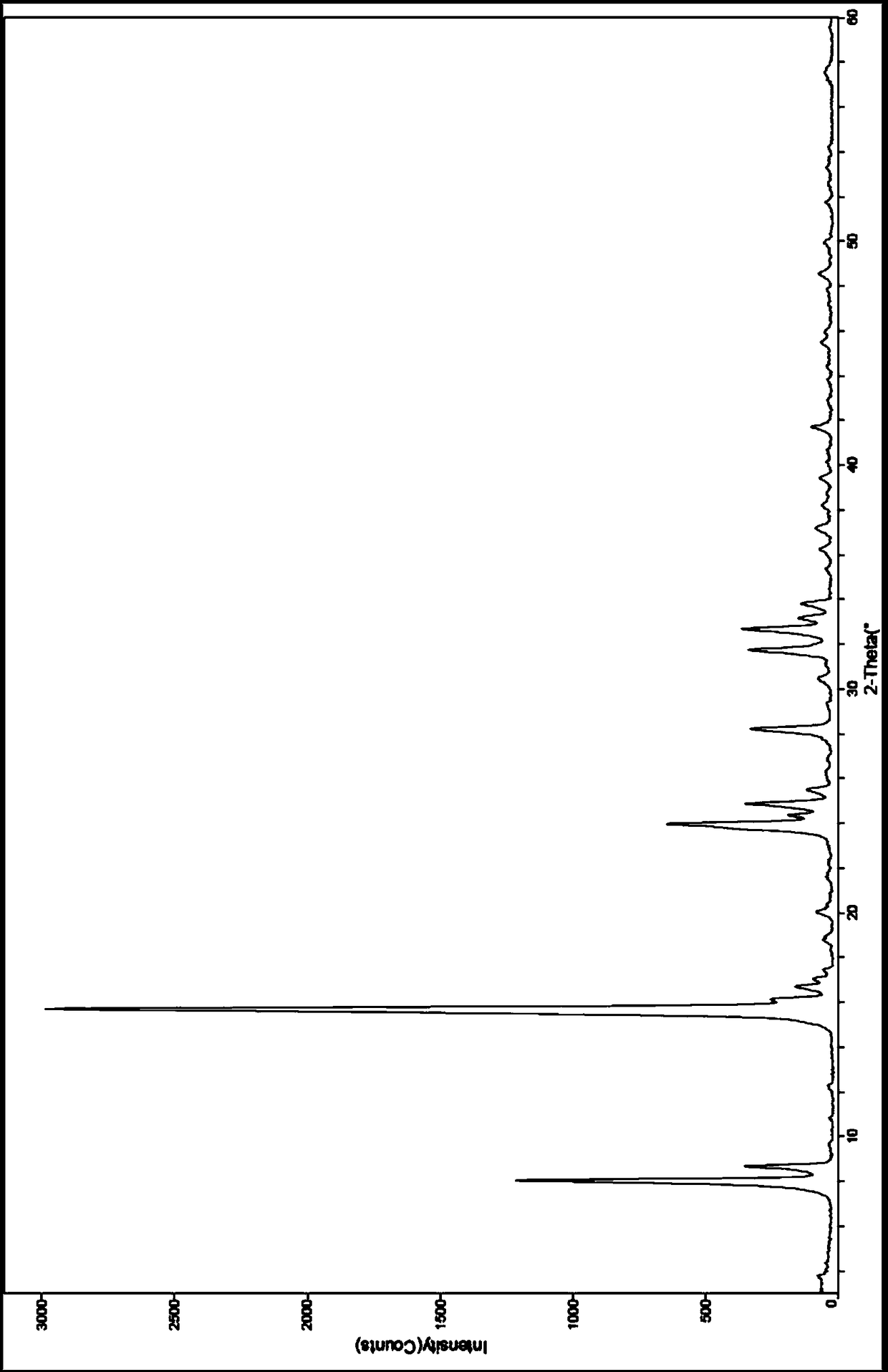
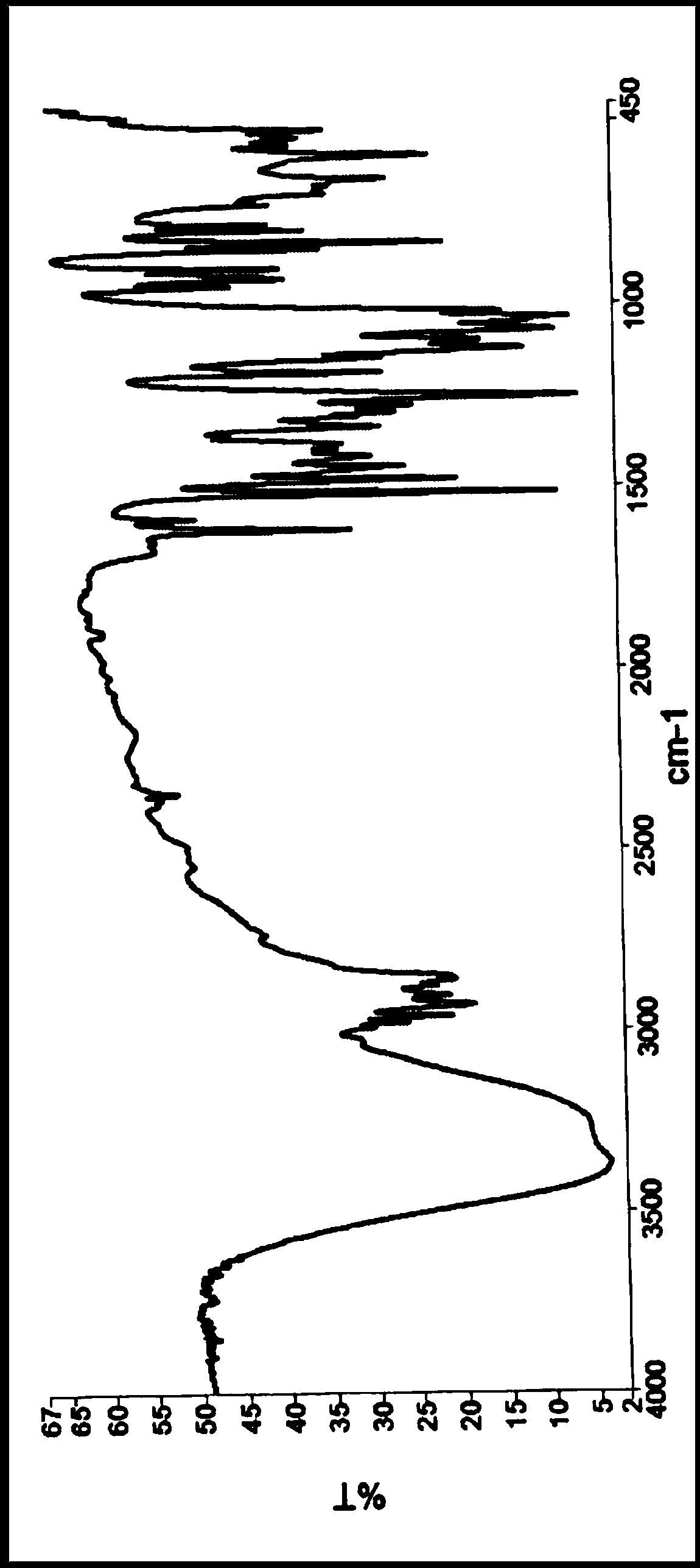
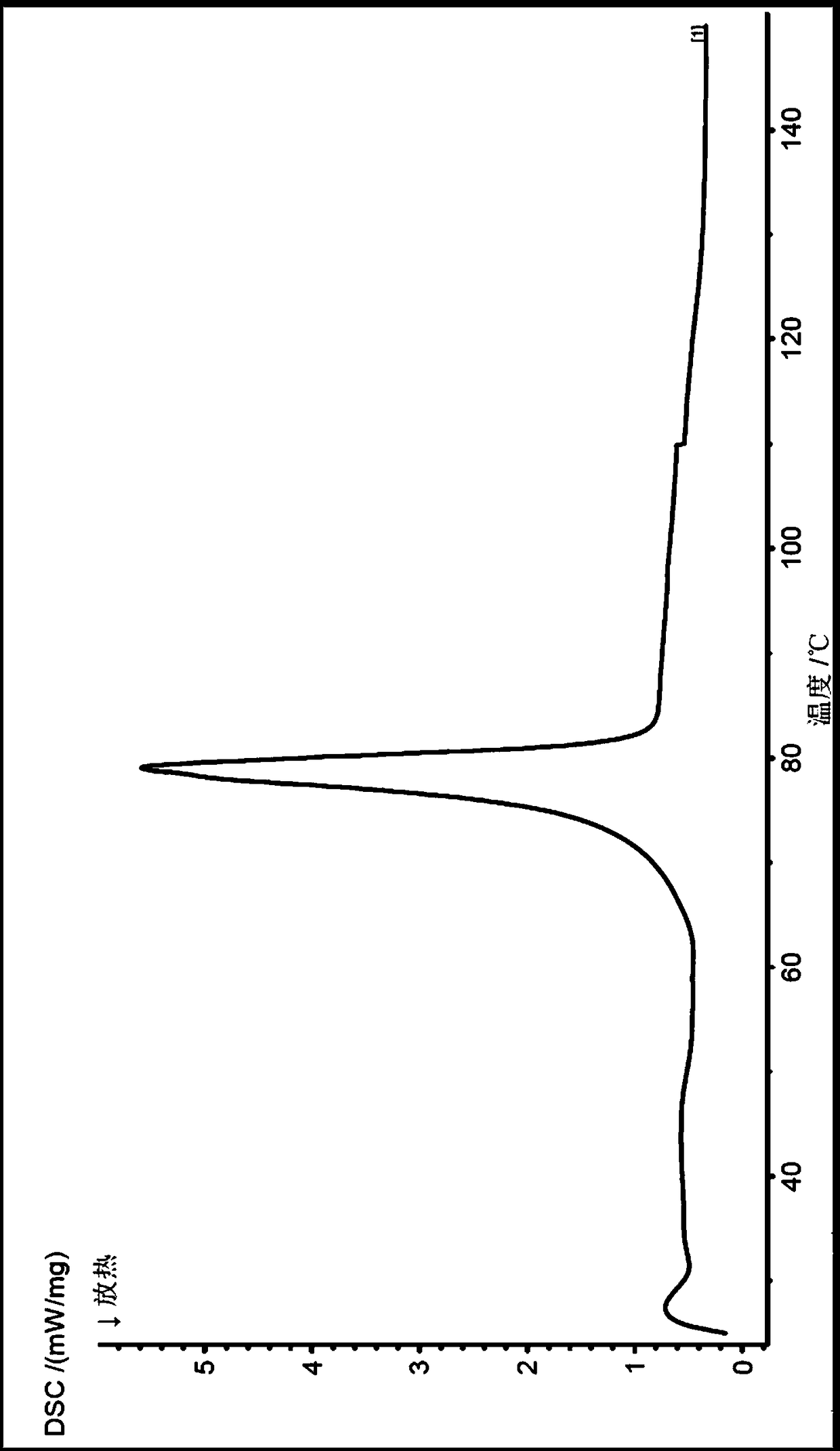
[0072] The X-ray powder diffraction, infrared, DSC and TGA spectra of this crystal form are detailed in Figure 1-4 , which is named Dapagliflozin·(S)-propylene glycol·hydrate crystal form D in the present invention.
Embodiment 2
[0074] Dissolve 0.1g of Dapagliflozin (S)-propylene glycol hydrate (HPLC>99%) in 30ml of dichloromethane, raise the temperature to 30°C, and continue stirring for 30min to dissolve; filter, and magnetically stir to control the stirring speed to 200rpm, The filtrate was cooled to 10°C, stirred and crystallized for 4 hours, filtered, and dried under vacuum (-0.1Mpa) at room temperature to obtain 0.040 g of crystals (HPLC>99%), which were confirmed to be Form D.
Embodiment 3
[0076] Dissolve 0.1g of Dapagliflozin (S)-propylene glycol hydrate (HPLC>99%) in 5ml of dichloromethane, heat up to 35°C, and continue to stir for 15min to dissolve; filter, and magnetically stir to control the stirring speed to 800rpm, The filtrate was cooled to 25°C, filtered, and dried at room temperature to obtain 0.065 g of crystals (HPLC>99%), which were confirmed to be Form D.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com