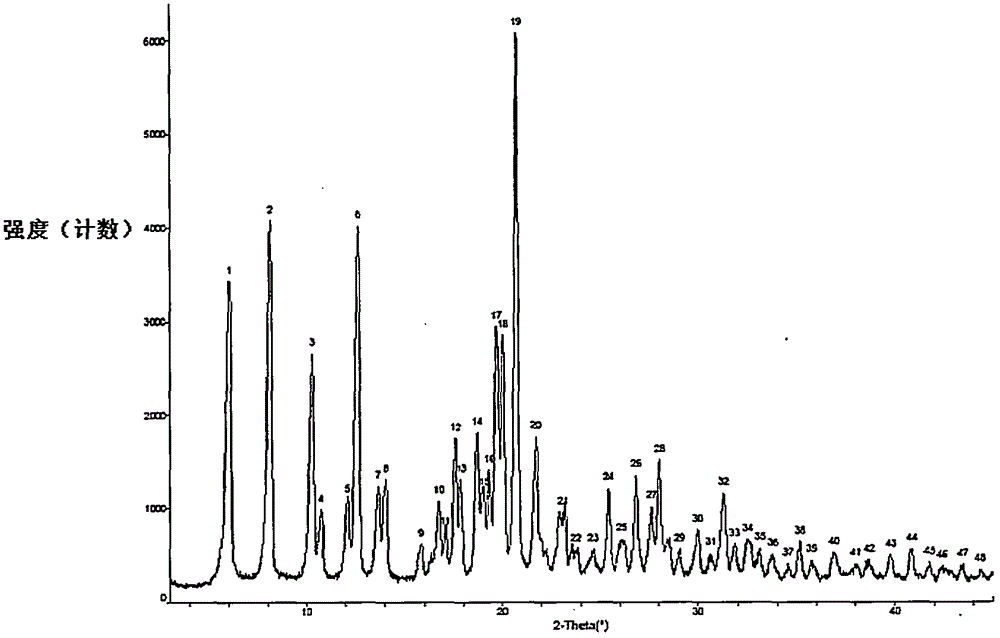
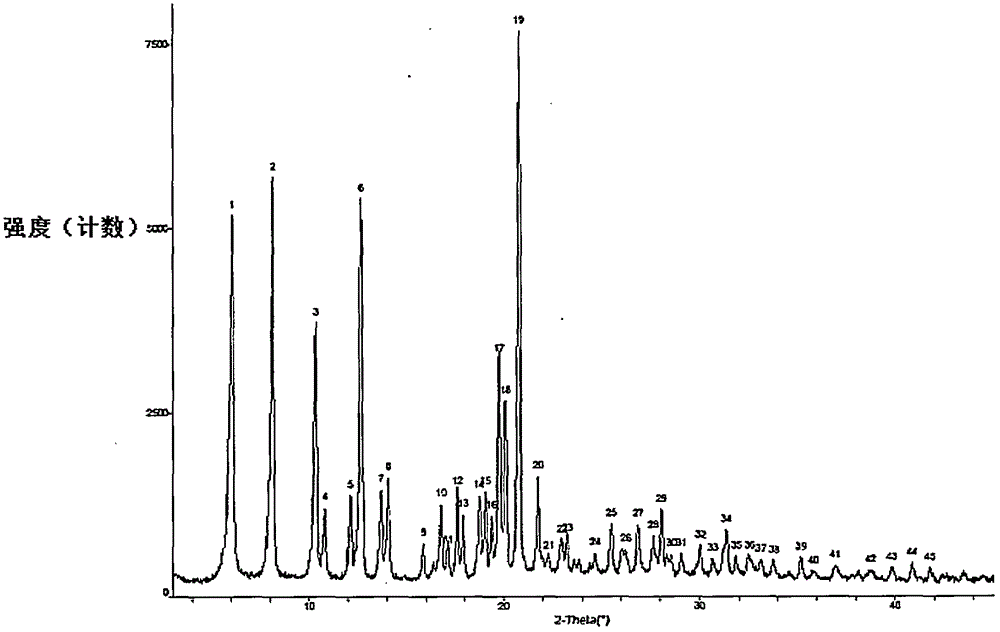
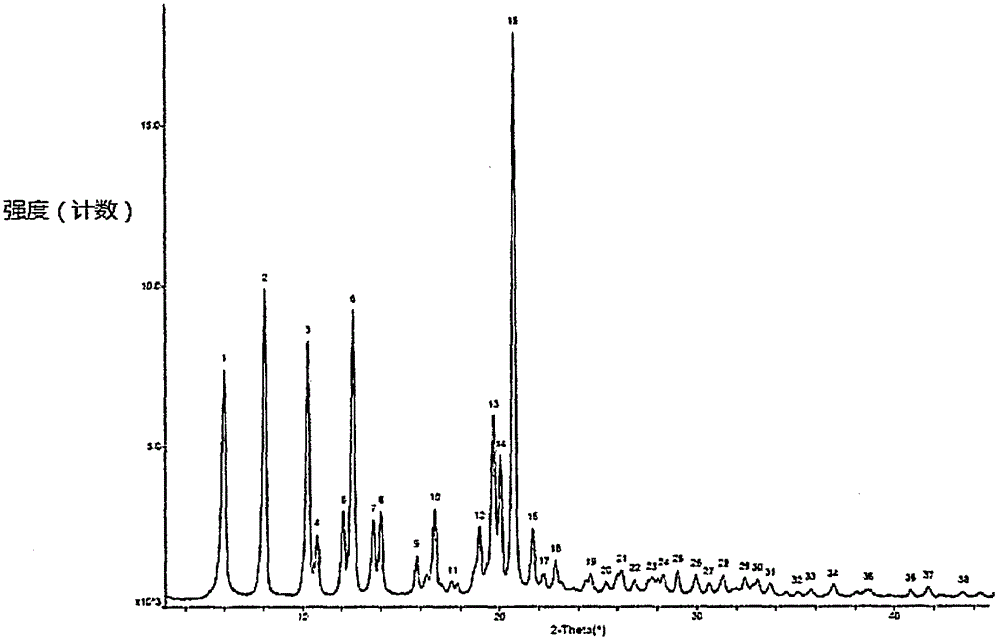
Preparation method of sofosbuvir crystal 6
A technology of febuvir crystal form and sofosbuvir, which is applied in the preparation of sugar derivatives, organic chemical methods, chemical instruments and methods, etc., can solve the problem that it is not easy to meet the standards of raw materials, unsuitable for industrial production, and cumbersome steps, etc. problem, to achieve simple and efficient crystallization process, short steps and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 10°C to 15°C, completely dissolve Sofosbuvir (amorphous, 5.0g) in ethanol (25mL) in a single-necked flask to obtain an ethanol solution of Sofosbuvir, and then transfer the ethanol solution of Sofosbuvir to Put it into the constant pressure dropping funnel and set aside.
[0039] Add water (100mL) to a three-necked flask equipped with mechanical stirring, turn on the mechanical stirring, and the stirring speed is 260 rpm. Slowly drop the funnel into the aqueous solution, which takes about 15 minutes, and a milky white suspension can be obtained after the drop is completed. Continue to stir at room temperature (10°C-35°C) for 12 hours to crystallize, filter with medium-speed filter paper, rinse the filter cake with water (15mL) once, and dry it in vacuum at 45°C-50°C for 16 hours (vacuum degree -0.008MPa), to obtain Febuvir product 4.8g, yield 96.0%, HPLC purity 99.75%, maximum single impurity 0.03%.
[0040] The crystal product obtained by drying is carried out to pow...
Embodiment 2
[0046] 5 ℃ ~ 10 ℃, sofosbuvir (crystalline form 1, 1.0kg) was completely dissolved in isopropanol (3L) in a single-necked flask to obtain the isopropanol solution of sofosbuvir, and then the sofosbuvir Transfer Wei's isopropanol solution to a constant pressure dropping funnel for later use.
[0047] Add water (22L) to the reactor equipped with mechanical stirring, turn on the mechanical stirring at a speed of 230 rpm, and at room temperature (10°C to 35°C), put the sofosbuvir isopropanol solution through constant pressure drop The funnel is slowly dropped into the aqueous solution, which takes about 60 minutes, and a milky white suspension can be obtained after the drop is completed. Continue to stir at room temperature (10°C-35°C) for 24 hours to crystallize, filter with 200-300-mesh filter cloth, rinse the filter cake with water (3L) once, and vacuum-dry at 45°C-50°C for 20 hours (vacuum degree -0.008MPa ), sofosbuvir product 967g, yield 96.7%, HPLC purity 99.97%, maximum s...
Embodiment 3
[0050] At 40°C to 45°C, completely dissolve Sofosbuvir (crystalline form 2, 100g) in isobutanol (1L) in a single-necked flask to obtain an isobutanol solution of Sofosbuvir, and then the Sofosbuvir Wei's isobutanol solution was transferred to a constant pressure dropping funnel for later use.
[0051] Add water (4L) to the reactor equipped with mechanical stirring, turn on the mechanical stirring, the rotating speed is 260 rpm, at room temperature (10 ° C ~ 35 ° C), the sofosbuvir isobutanol solution is passed through the constant pressure drop Slowly drop the funnel into the aqueous solution, which takes about 30 minutes, and a milky white suspension can be obtained after the drop is complete. Continue to stir at room temperature (10°C-35°C) for 24 hours to crystallize, filter with 200-300-mesh filter cloth, rinse the filter cake with water (100mL) once, and vacuum-dry at 45°C-50°C for 20 hours, vacuum degree -0.008MPa 95.1 g of the sofosbuvir product was obtained, the yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com