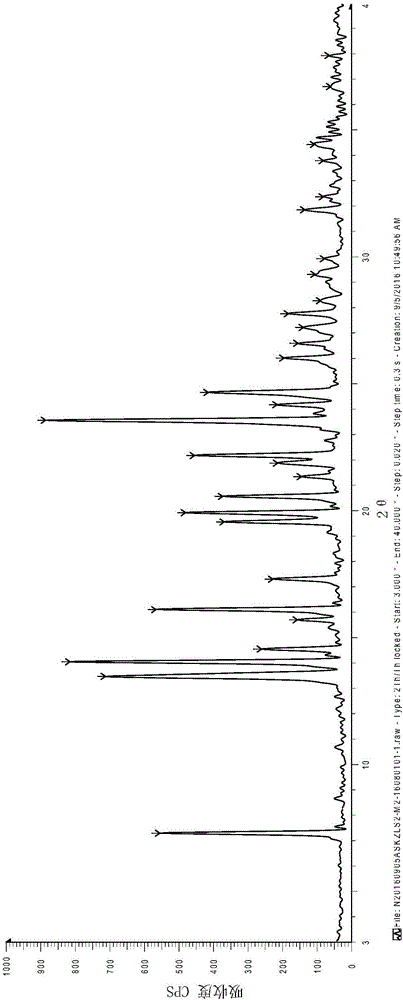
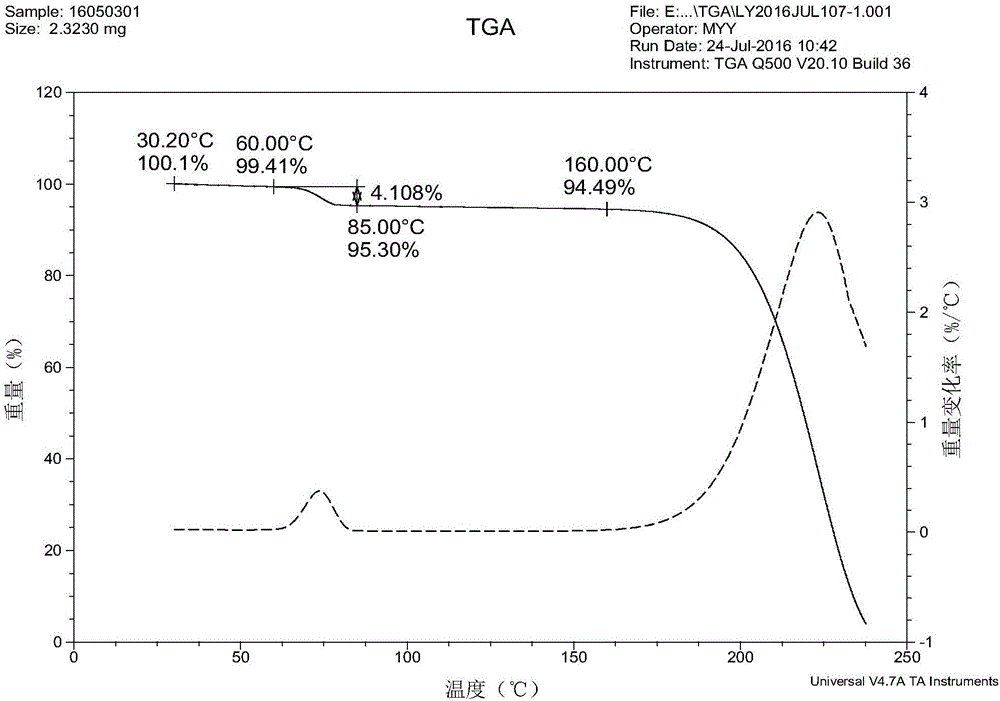
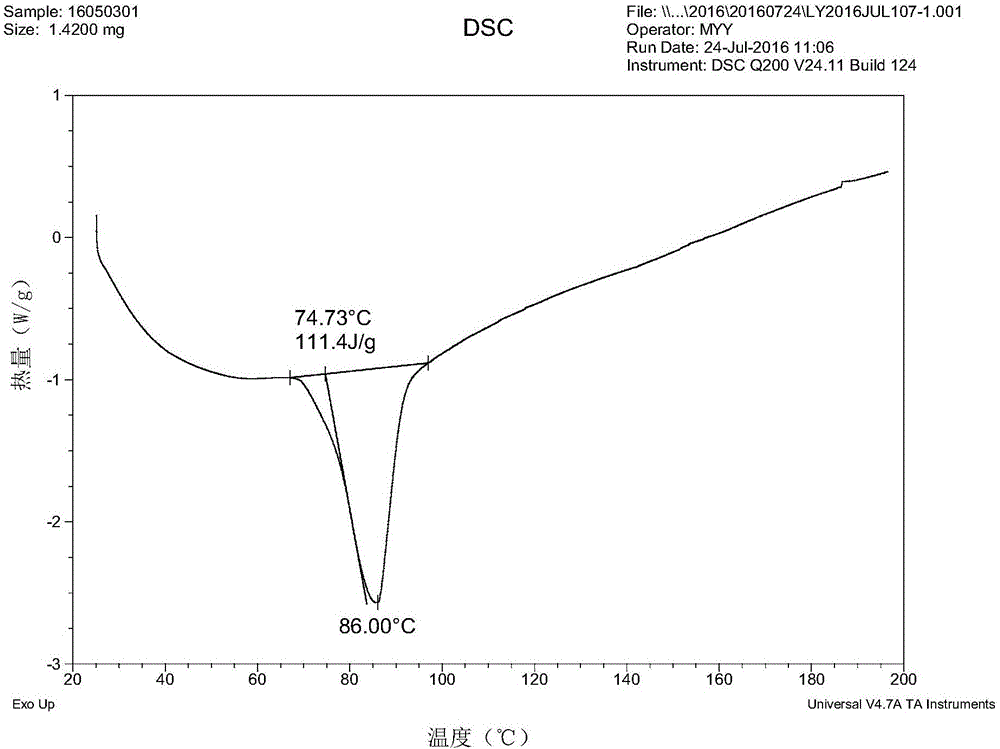
Isavuconazole monohydrate crystal form and preparation method thereof
A technology of isavuconazole monohydrate and monohydrate, which is applied in the field of pharmacy, can solve the problems of complex process technology, complicated preparation process, and long time consumption, and achieve simple crystallization process, strong operability, and favorable industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of formula 3 compound
[0055] In the 5L three-necked round bottom reaction flask, add formula 2 compound successively, i.e. diethyl dithiophosphate (804g), formula 1 compound (300g), isopropanol (1.5L) and purified water (194ml), stir and heat up To reflux reaction (oil temperature 85°C), internal temperature 78°C, keep reflux, gradually a large amount of H 2 S gas is released until the temperature stabilizes at an internal temperature of 78°C, without a large amount of gas being released, and the temperature is kept stirring. After 15 hours, sampling and monitoring were performed, and TLC showed that SM1 spots disappeared and new spots were formed.
[0056] Cool down to below 20°C, add DCM (1.5L), purified water (1.5L). Slowly add 10wt% NaOH solution (about 2.3 L) dropwise under stirring to adjust the pH value to 7-8, and the system turns yellow and gray during the addition. Separate and extract, and collect the lower DCM phase. The aqueous phase w...
Embodiment 2
[0058] The preparation of formula 5 compound
[0059] In the 5L three-necked round-bottom reaction flask, add the formula 3 compound (300g) that embodiment 1 prepares, the formula 4 compound namely 2-bromo-4 cyano-acetophenone (215g) and 95% ethanol (1.5L), stir Lower the temperature to 85°C oil temperature and 76°C internal temperature. During the heating process, the color of the system turns yellow-green, and keep stirring for 2 hours. Sampling and monitoring, TLC showed that M1 spots disappeared and new spots were generated.
[0060] Cool down to below 20°C, under stirring, dissolve saturated NaHCO 3 Slowly add the solution into the reaction solution, adjust the pH value to 4-5, white solid precipitates, continue to stir until the crystals are completely precipitated, then add water dropwise into the system, so that the total water volume reaches 1.5L. After stirring for 2 h, it was filtered, and the solid was washed with a mixed solvent of EtOH: water = 1:1. The result...
Embodiment 3
[0063] The preparation of formula 5 compound
[0064] In the 5L three-necked round-bottom reaction flask, add the formula 3 compound (300g) that embodiment 1 prepares, the formula 4 compound namely 2-bromo-4 cyano-acetophenone (215g) and 95% ethanol (1.5L), stir Lower the temperature to 85°C oil temperature and 76°C internal temperature. During the heating process, the color of the system turns yellow-green, and keep stirring for 2 hours. Sampling and monitoring, TLC showed that M1 spots disappeared and new spots were generated.
[0065] Cool down to below 20°C, under stirring, dissolve saturated NaHCO 3 Slowly add the solution into the reaction solution, adjust the pH value to 4-5, and then slowly pour the reaction solution into purified water (1.5L), a large amount of white solids are precipitated, and after suction filtration, the filter cake is naturally aired to semi-dry, and the The drying time is about 16 hours. Add DCM (1.2L) and purified water (600ml) to the semi-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com