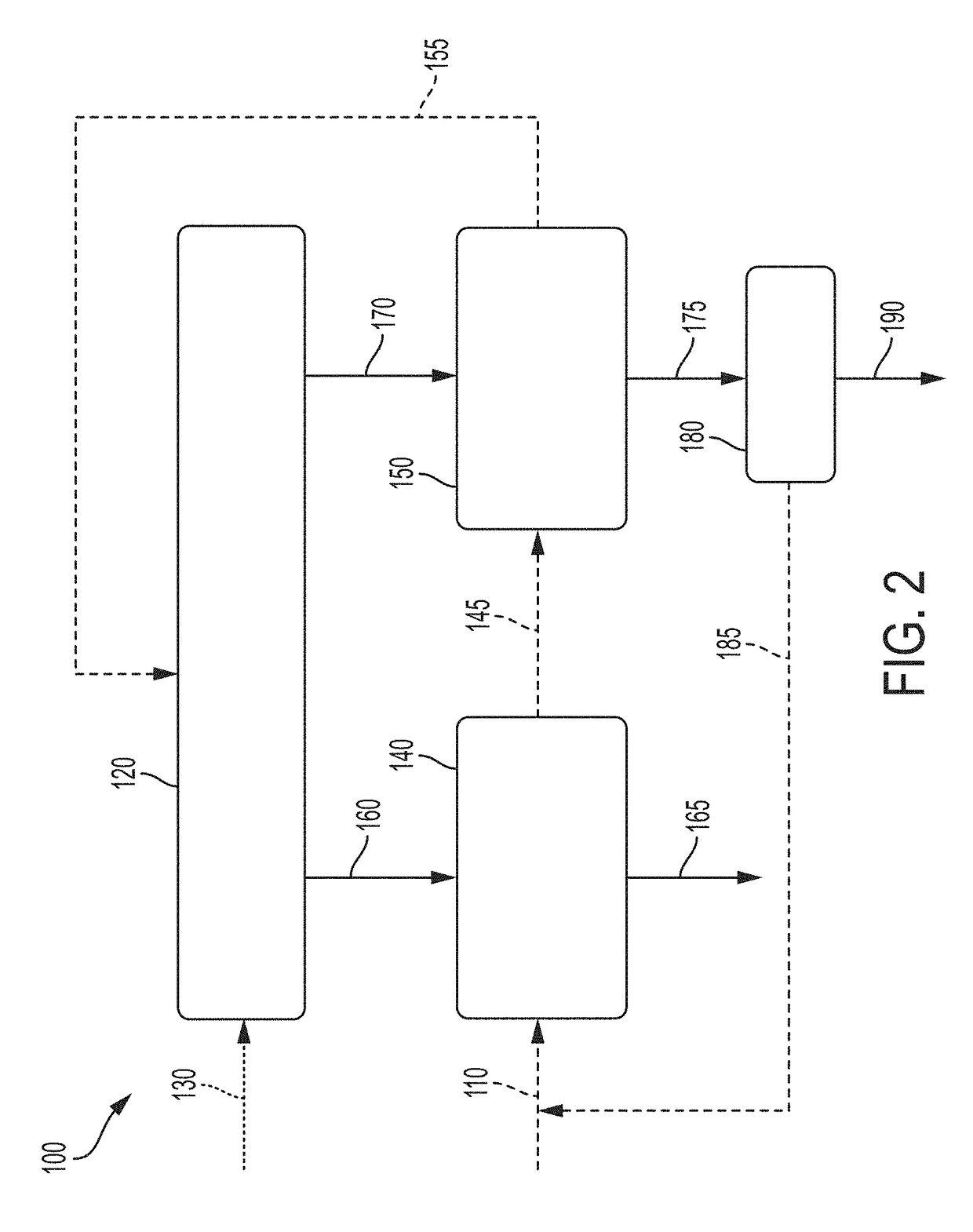
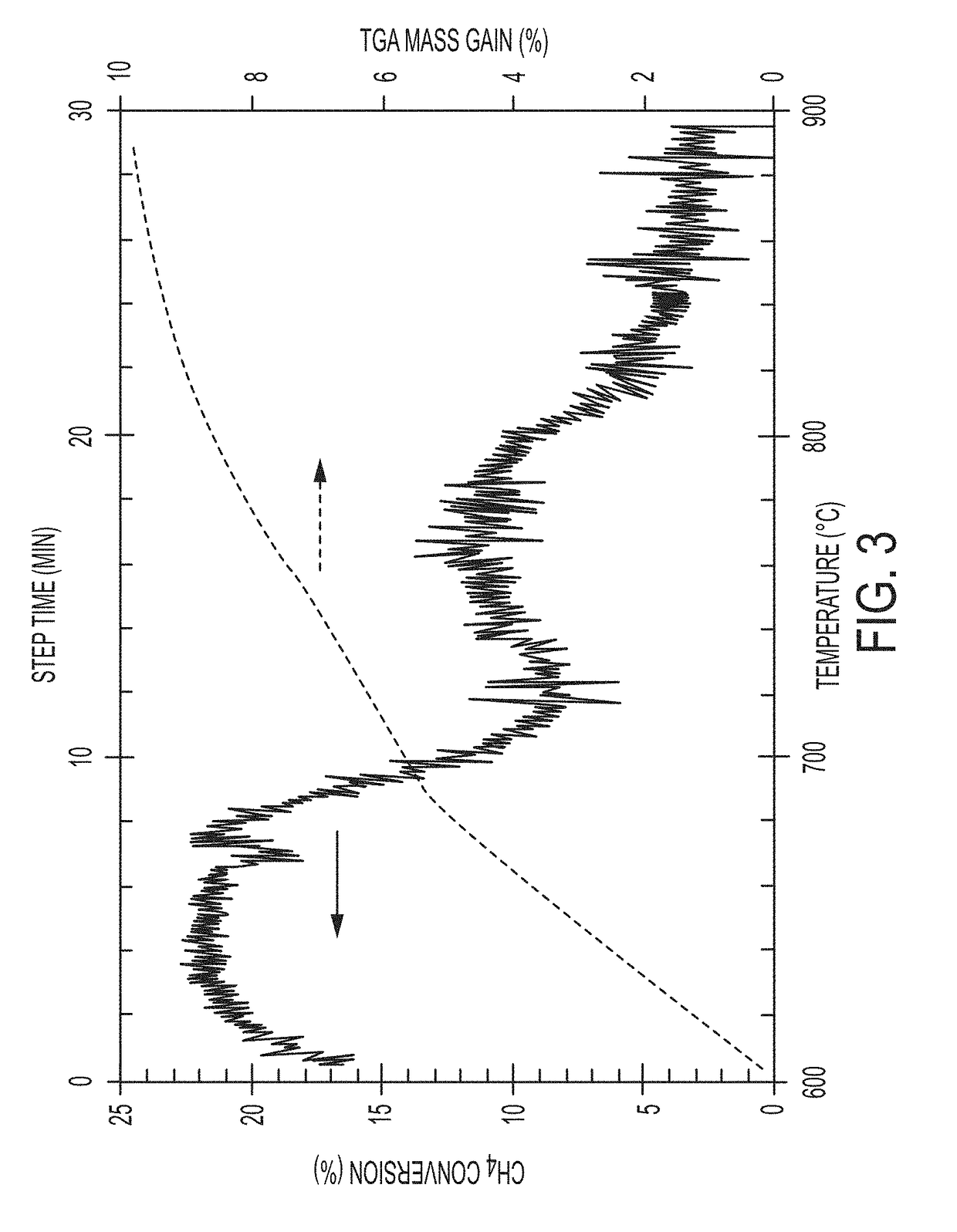
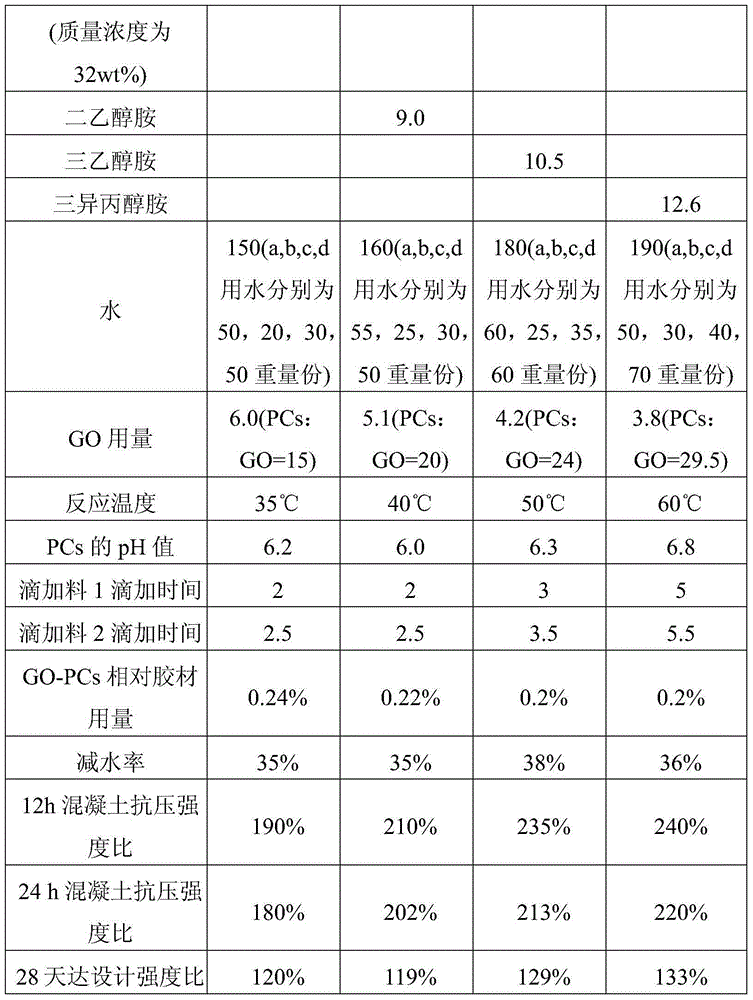
Patents
Literature
217results about How to "Prevent coalescence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Low level cure transfuse assist for printing with radiation curable ink
InactiveUS7270408B2High viscosityPrevent coalescenceMeasurement apparatus componentsDuplicating/marking methodsEngineeringViscosity
The method of forming an image formed of low viscosity ink on a recording medium includes ejecting the low viscosity ink from a printer head in the form of droplets onto an intermediate transfer medium to form the image, partially curing the image on the intermediate transfer medium, transferring the partially cured image onto the recording medium, and further curing the partially cured image on the recording medium to create a hardened image.
Owner:XEROX CORP
Method for preparing edible grease gel with Pickering emulsion as template
ActiveCN105994698AHigh strengthInhibition of oil-water phase separationEdible oils/fatsChemistryOil in water
The invention relates to a method for preparing edible grease gel with a Pickering emulsion as a template. Firstly, an oil-in-water type emulsion with soybean isolate protein nanoparticles as an emulsifier and xanthan gum as a gel enhancer is prepared, moisture in the emulsion is removed with a drying means, and the edible grease gel containing little water is obtained. By comparison with a traditional emulsion, particles can be adsorbed to an oil / water interface of the oil-in-water type emulsion irreversibly, accordingly, coalescence and flocculation of liquid drops and curing of Ostwald are effectively inhibited, and the emulsion can keep stable for months or years. With the adoption of the preparation method, grease which does not contain trans-fatty acids and low-content saturated fatty acids can be obtained and can replace part of solid or semi-solid fat in food. The method has the advantages that operation is simple and convenient, raw materials are safe and environment-friendly and the like.
Owner:河南福美生物科技有限公司
Feed-dispersion system for fluid catalytic cracking units
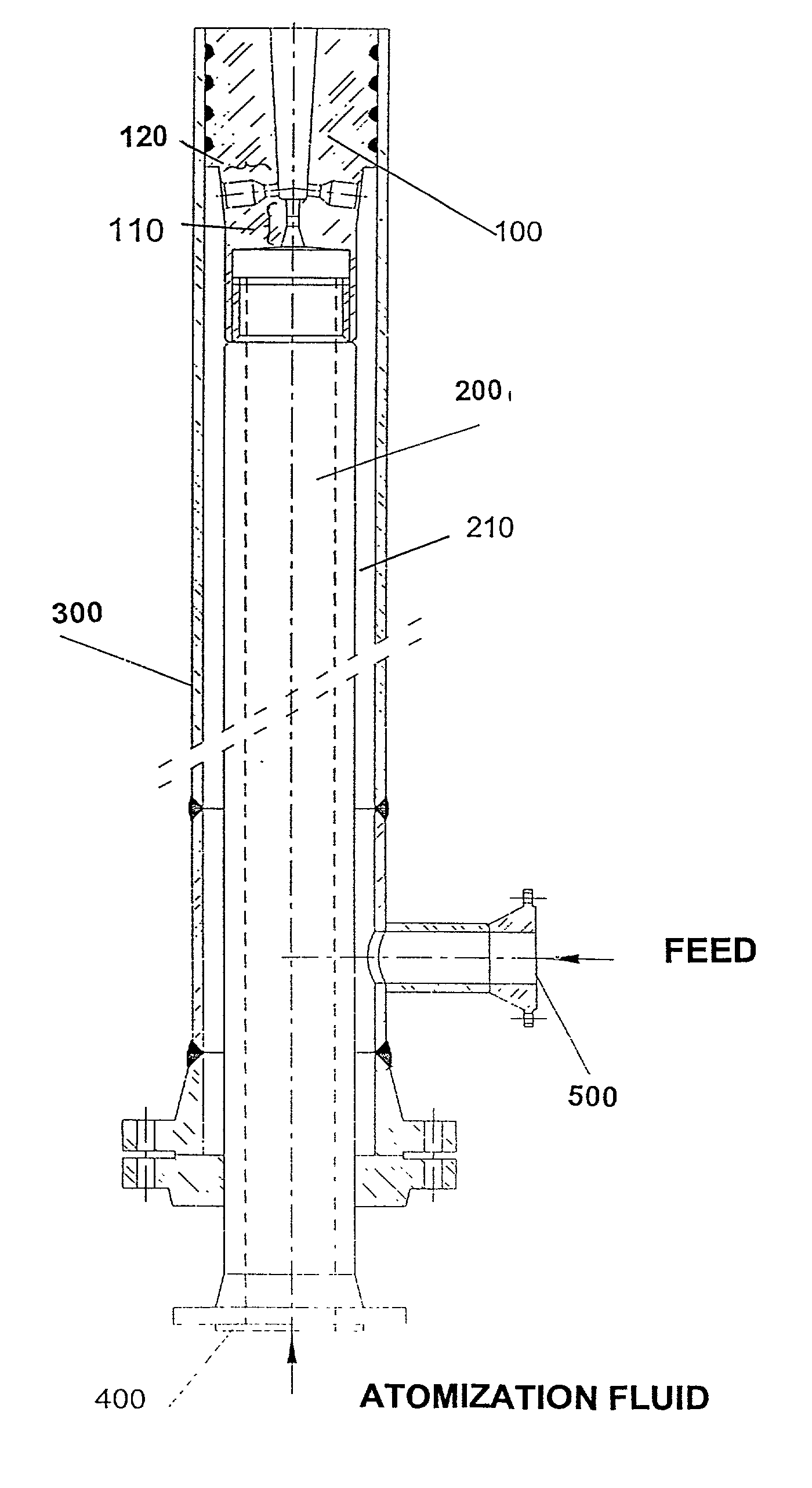
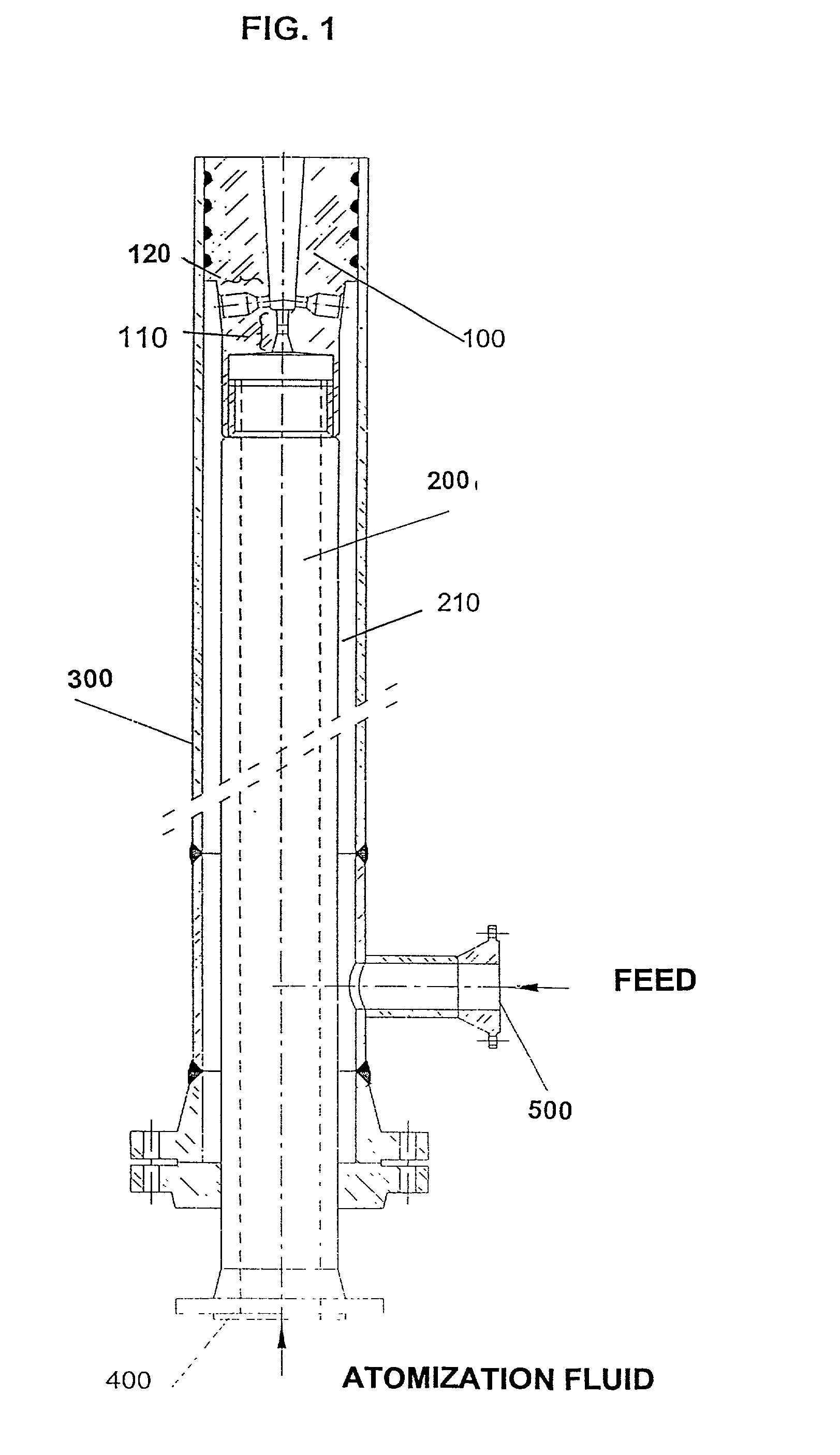
InactiveUS6936227B1Great feed conversionReduce pump powerCatalytic crackingSpray nozzlesEngineeringGuide tube
A feed-dispersion system for hydrocarbon feeds of fluid catalytic cracking units is described, which comprises:a feed-injection system made up of two concentric conduits, where the atomization fluid flows through the inner conduit, while the liquid feed flows through the annular space formed by the outer surface of the inner conduit and the inner surface of the outer conduit;an atomization unit having nozzles arranged in rows, with one row having central nozzles connected to the inner conduit for atomization fluid, and two or more rows of side nozzles, connected to the outer feed conduit, the central nozzles and side nozzles of the atomization unit being geometrically placed so that the energy of the atomization fluid is fully transferred by contact to the flow of feed, this resulting in the complete atomization of the feed;a mixing chamber formed by the edges of the central nozzles, the dimensions of which are able to prevent the coalescence of the formed oil droplets.
Owner:PETROLEO BRASILEIRO SA (PETROBRAS)
Reflective film, reflection type liquid crystal display, and sputtering target for forming the reflective film
InactiveUS7022384B2Improve antioxidant capacityImprove reflectivityLiquid crystal compositionsMirrorsLiquid-crystal displayRare earth
A reflective film used as a reflective electrode or a reflector, comprising an Ag-based alloy containing rare earth metals. The reflective film has high reflectivity and high durability.
Owner:KOBE STEEL LTD
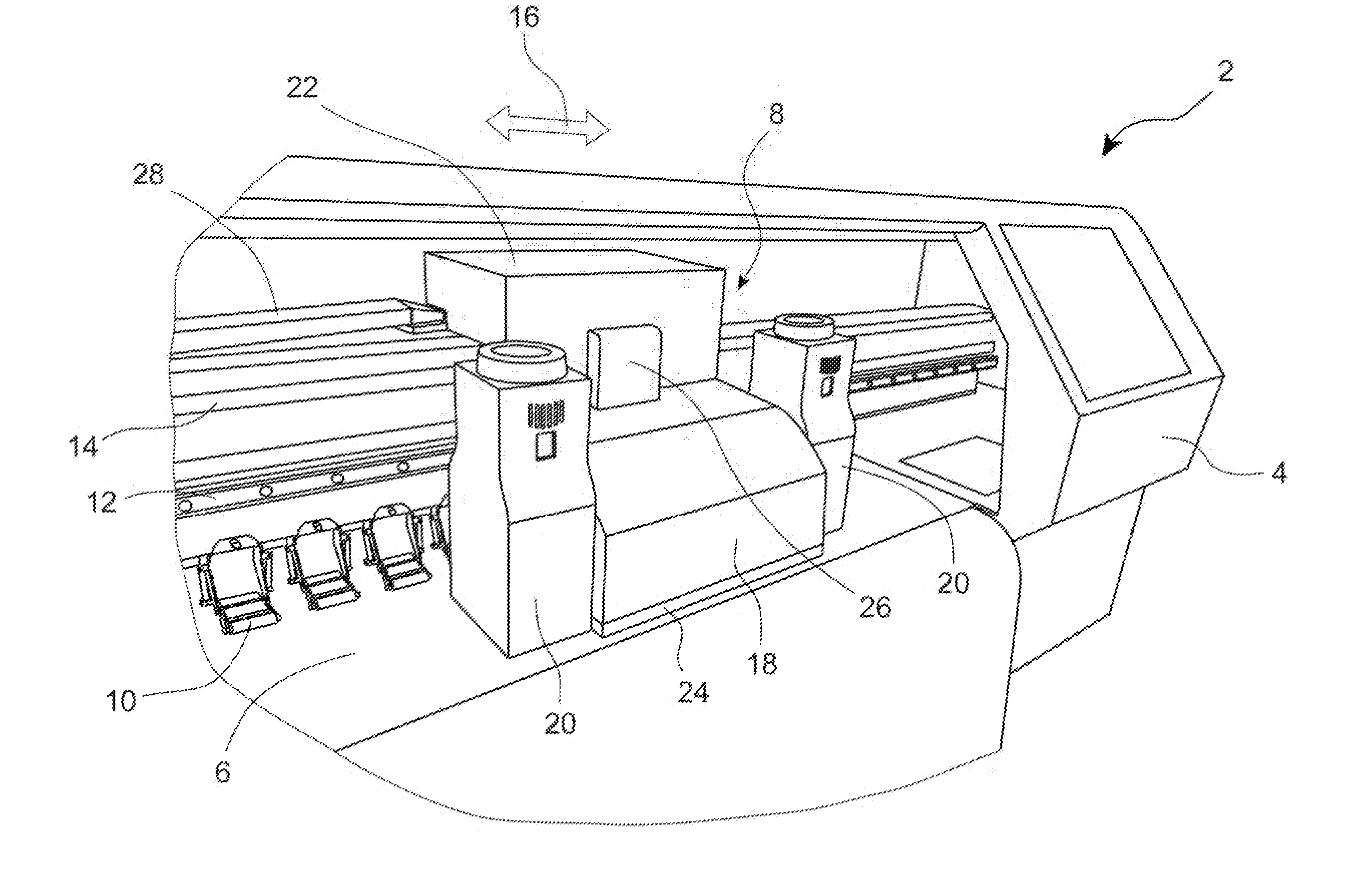
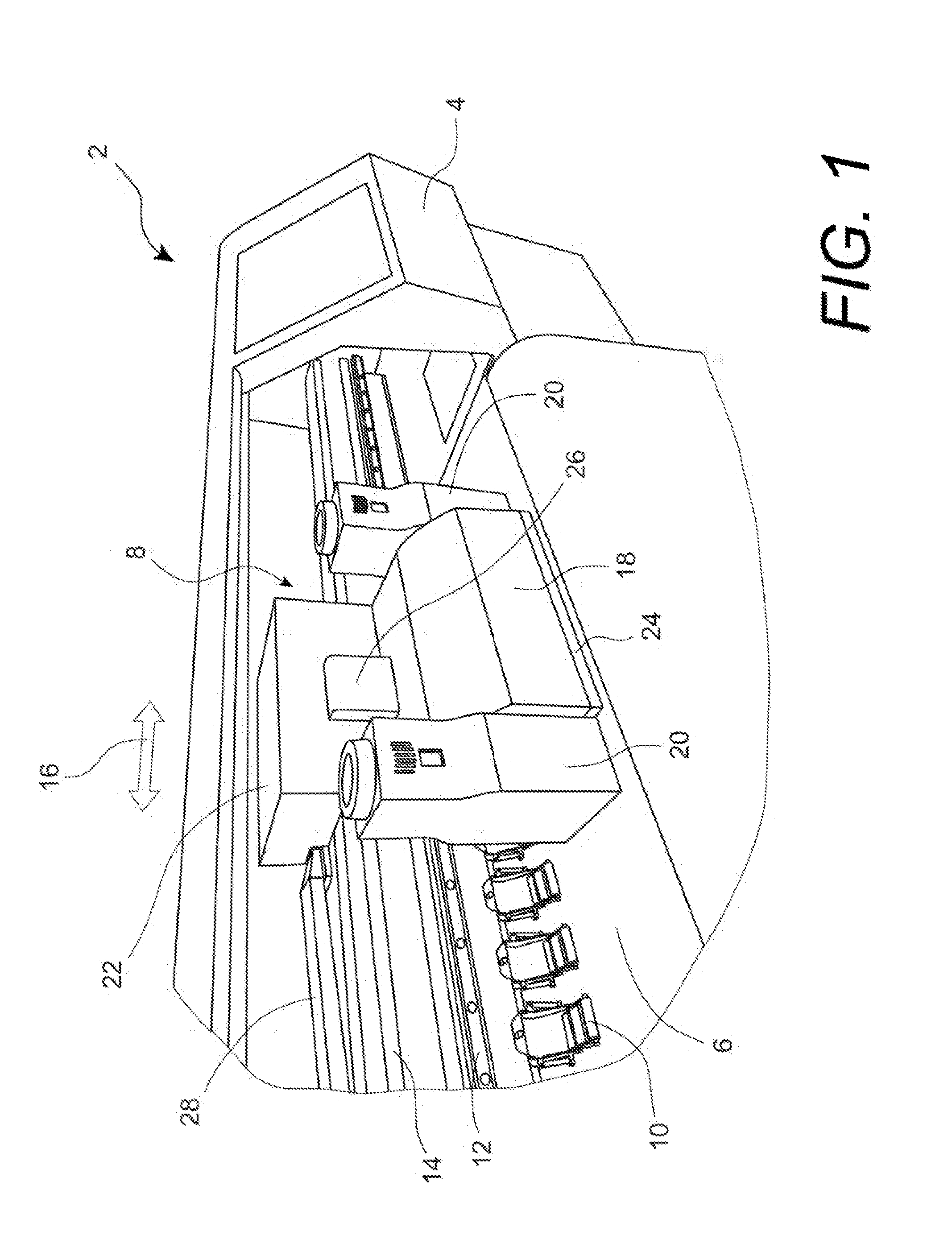
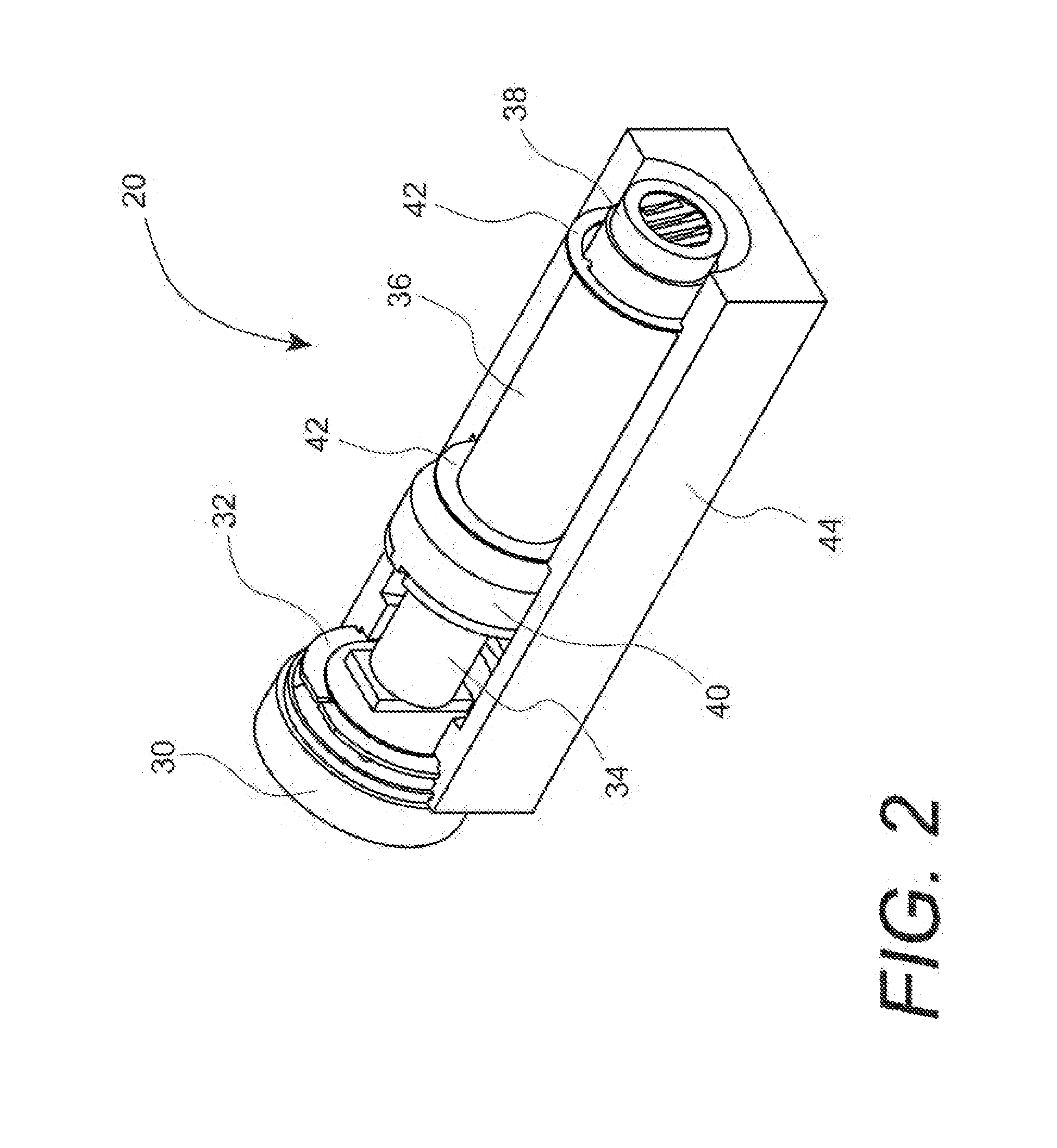
Apparatus and method for printing sharp image in an inkjet printer
InactiveUS20130215203A1Cost advantagePrevent coalescenceOther printing apparatusImaging qualityEngineering
An apparatus and method to heat media in order to achieve rapid ink drying for sharp image quality is disclosed. The apparatus comprises a media heating means to heat media approximate the print zone to prevent coalescence and inter-color bleeding, and a printhead cooling means to cool the printhead so that the printhead temperature is maintained below an upper printhead temperature limit to minimize clogged nozzles. The preferred embodiment includes a hot air blower attached to the movable carriage to impinge heated air directly on to the media surface, and circulating a liquid coolant in a fluid channel built in a printhead plate conductively connected to the printhead.
Owner:MEIJET COATING & INKS
Fluidized bed reactor
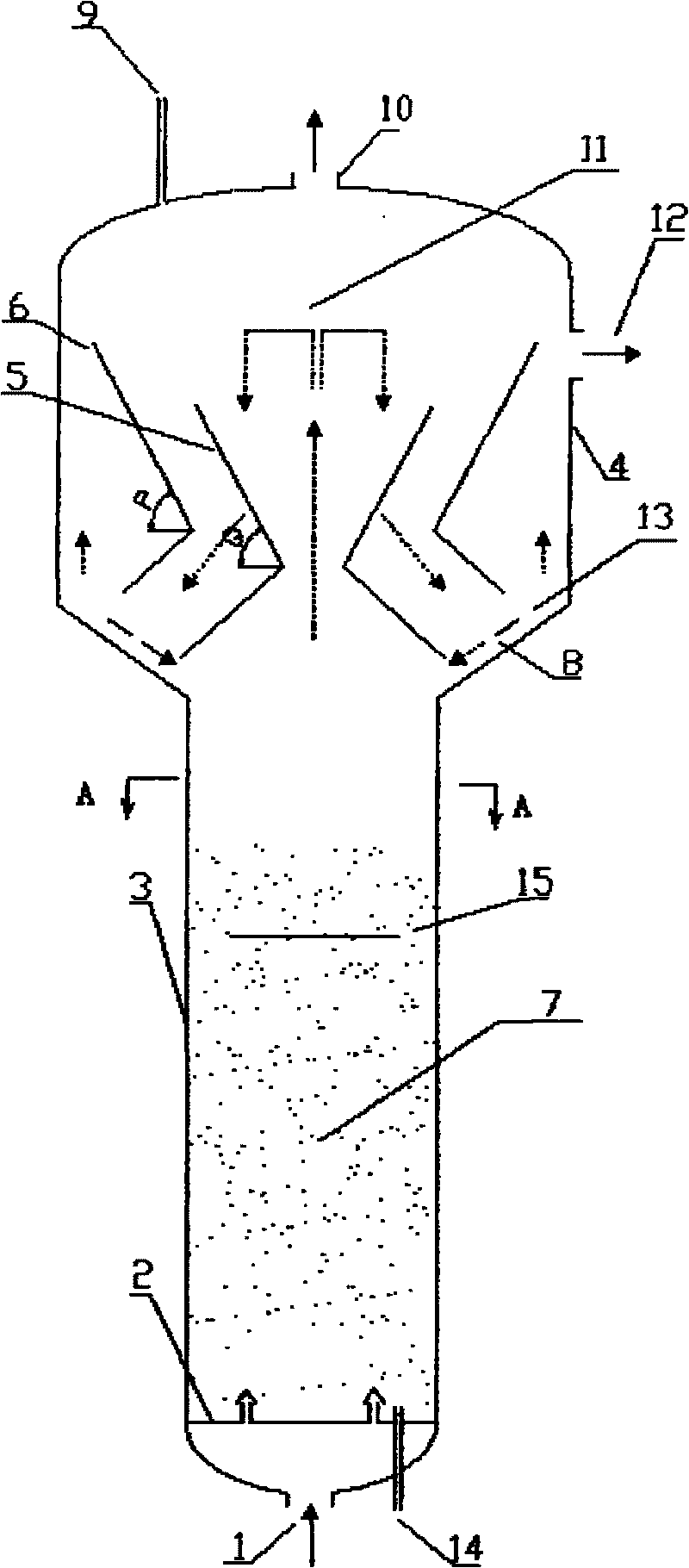
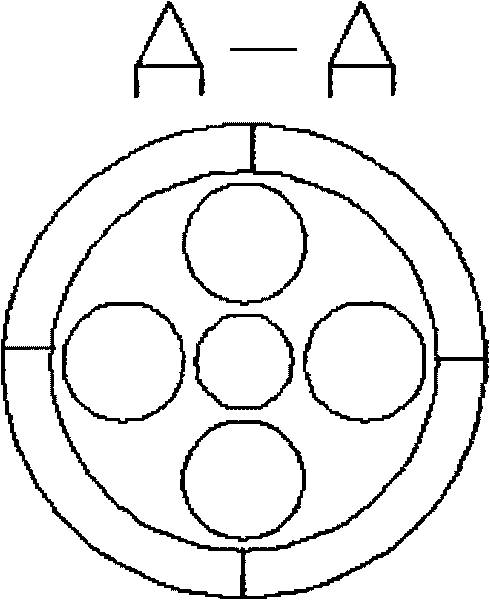
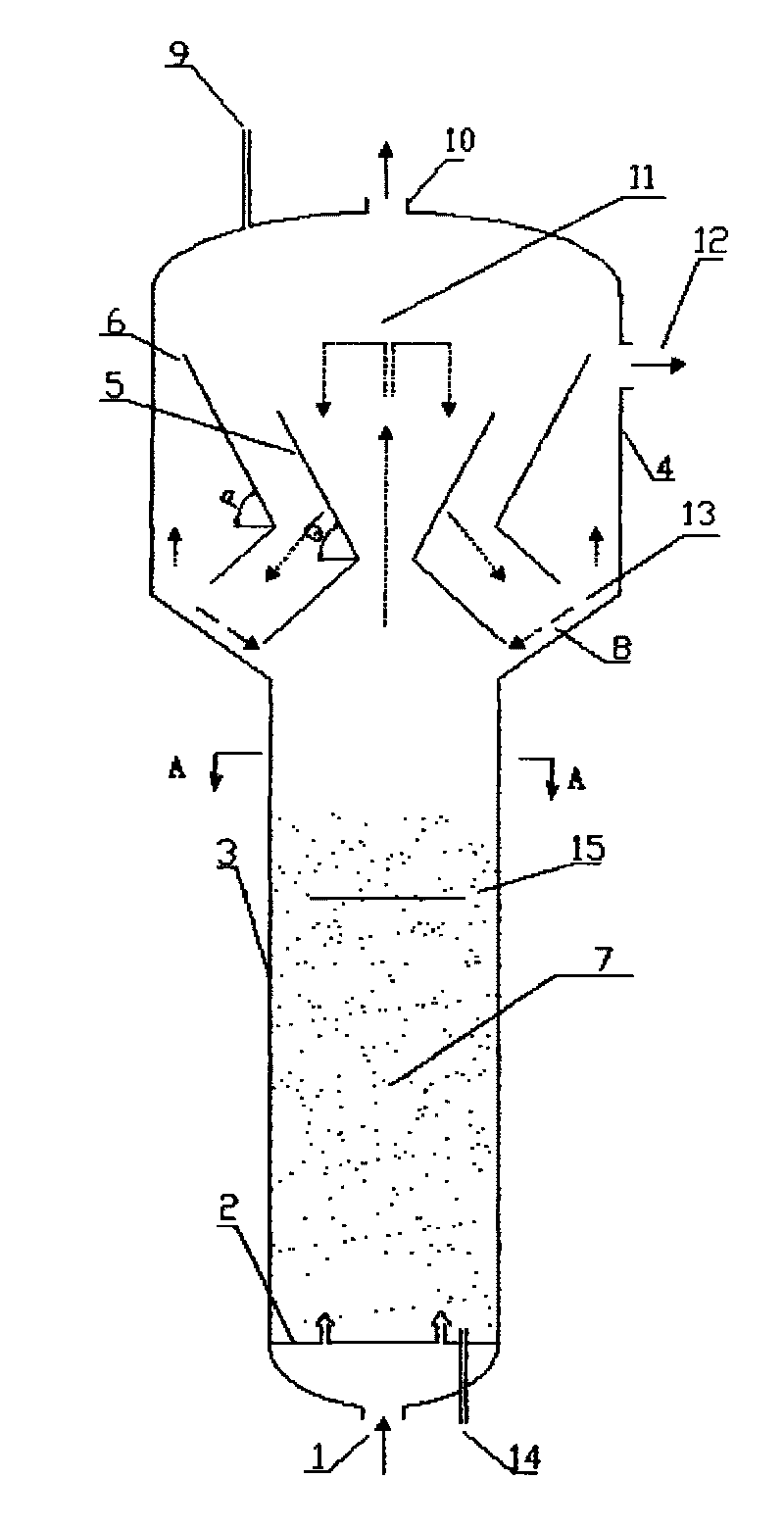
ActiveCN101721960AGreat operating flexibilityEfficient separationChemical/physical processesChemical reactionFluidized bed
The invention discloses a fluidized bed reactor which comprises a reactor shell and a three-phase separator, the three-phase separator is arranged at the upper part inside the reactor shell and is of a sleeve structure comprising an inner sleeve and an outer sleeve, the upper ends and lower ends of the inner sleeve and the outer sleeve are both of opening structures, the inner sleeve and the outer sleeve are respectively composed of an upper section and a lower section, the upper sections of the inner sleeve and the outer sleeve are in reverse frustum structures, and the lower sections of the inner sleeve and the outer sleeve are in frustum structures. By designing a novel three-phase separating structure, the invention can further improve separating effect, reduce catalyst carrying-out amount, and enhance the operation elasticity of the three-phase separator. The reactor is mainly applicable to chemical reactions between liquid and gas substances of different types and solid particles under contact condition, and has the advantages of large catalyst inventory, high reactor use ratio, simple structure, easy operation and the like.
Owner:CHINA PETROLEUM & CHEM CORP +1
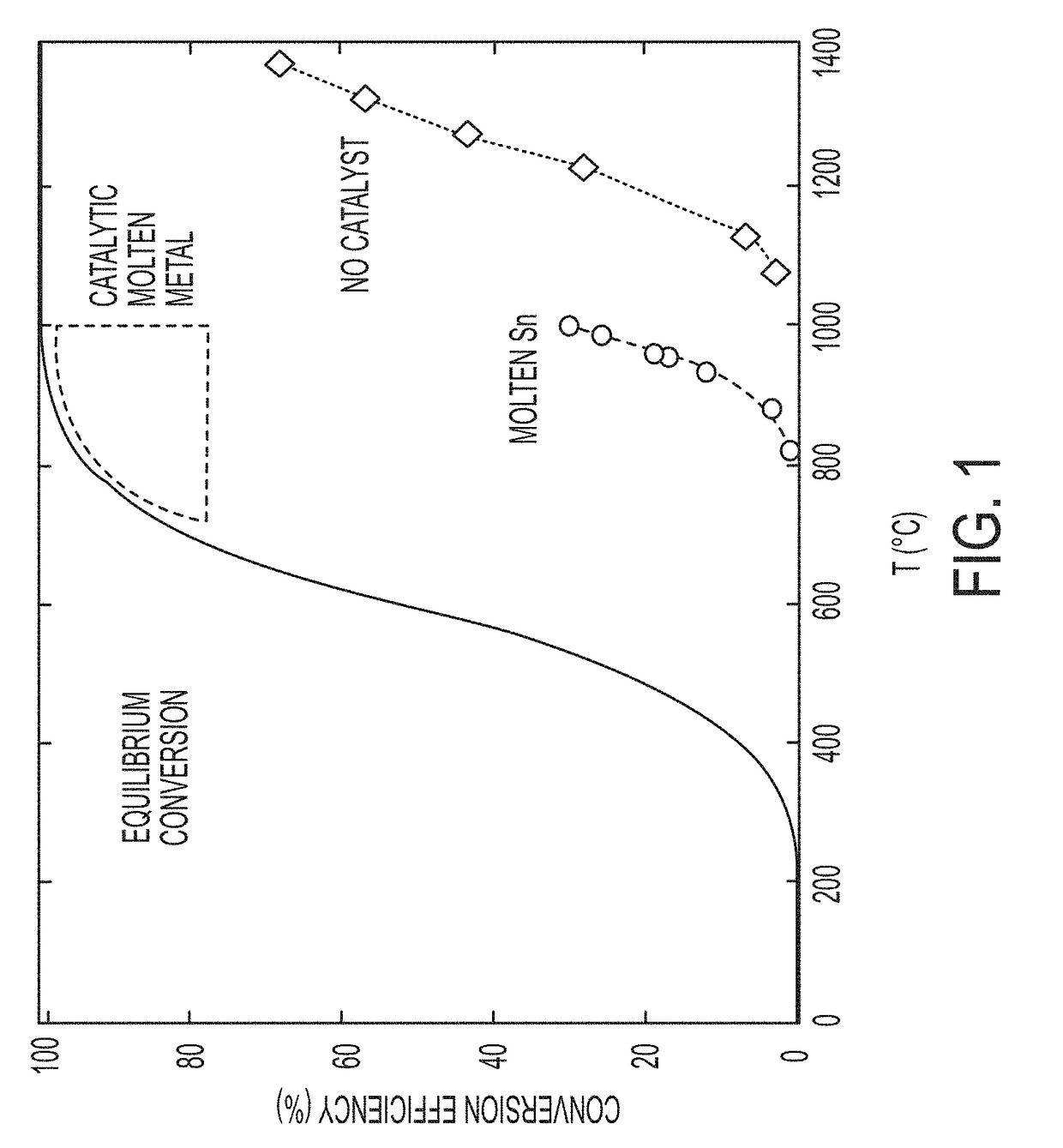
System and method for pyrolysis using a liquid metal catalyst
ActiveUS20190055173A1Elongated bubble shapeLarge specific surface areaPigmenting treatmentGraphiteSolid carbonHydrogen
A process for decomposing a hydrocarbon-containing composition includes feeding the hydrocarbon-containing composition to a reactor containing a catalytically active molten metal or a catalytically active molten metal alloy, wherein the metal or alloy catalyzes a decomposition reaction of the hydrocarbon-containing composition into a hydrogen-rich gas phase and a solid carbon phase. The solid carbon phase is insoluble in the metal or alloy. The process may be a continuous process.
Owner:XEROX CORP
Low level cure transfuse assist for printing with radiation curable ink
InactiveUS20060158496A1Low viscosityHigh viscosityMeasurement apparatus componentsDuplicating/marking methodsEngineeringViscosity
The method of forming an image formed of low viscosity ink on a recording medium comprises ejecting the low viscosity ink from a printer head in the form of droplets onto an intermediate transfer medium to form the image, partially curing the image on the intermediate transfer medium, transferring the partially cured image onto the recording medium, and further curing the partially cured image on the recording medium to create a hardened image.
Owner:XEROX CORP
Multifunctional enhancement-type concrete admixture and preparation method thereof
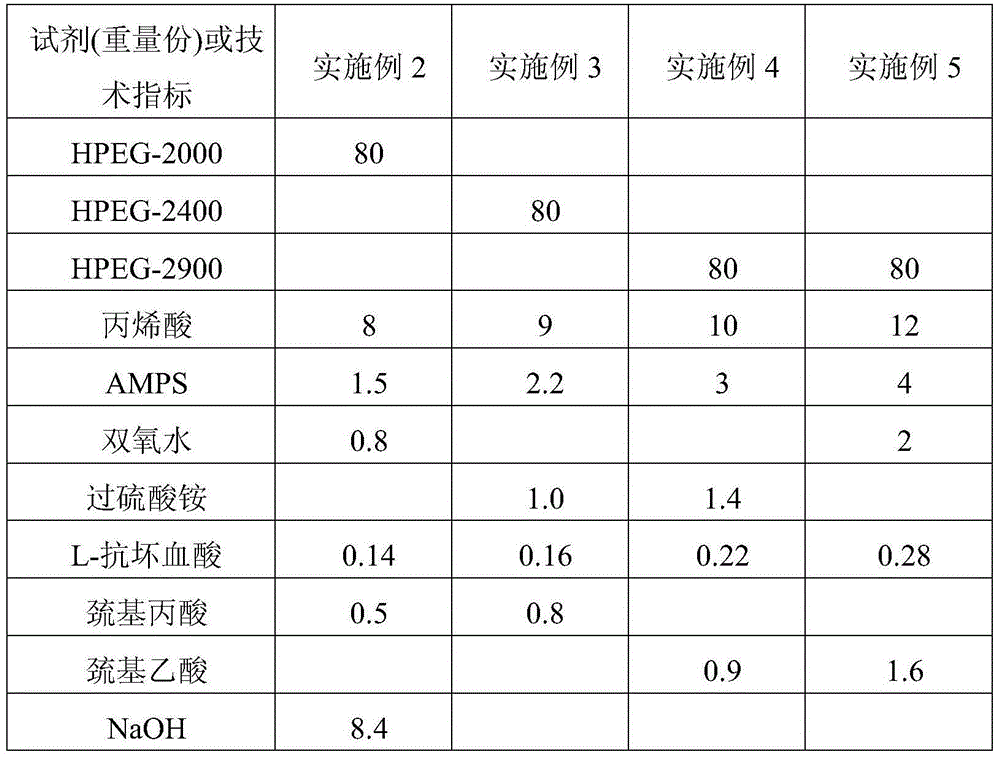
The invention relates to a multifunctional enhancement-type concrete admixture and a preparation method thereof. The concrete admixture is composed of an early-strength polycarboxylic acid water reducing agent (PCs) and nano graphene oxide (GO). The mass ratio of the solid to the GO in the early-strength water reducing agent (PCs) is (10-30):1. The early-strength polycarboxylic acid water reducing agent (PCs) is composed of the following components in parts by weight: 80 parts of unsaturated polyether, 8-12 parts of acrylic acid, 1.5-4 parts of monomer with functional groups, 0.5-1.8 parts of chain-transfer agent, 0.6-2 parts of initiator, 0.12-0.3 part of reducer, 8.4-13.2 parts of neutralizing alkali liquor and 150-200 parts of water. The admixture has an obvious enhancement action on concrete, implements high-strength long-service-life green concrete, and has important meanings in the field of application of high-strength high-performance concrete.
Owner:JIANGSU CHINA RAILWAY ARIT NEW MATEIRALS CO LTD
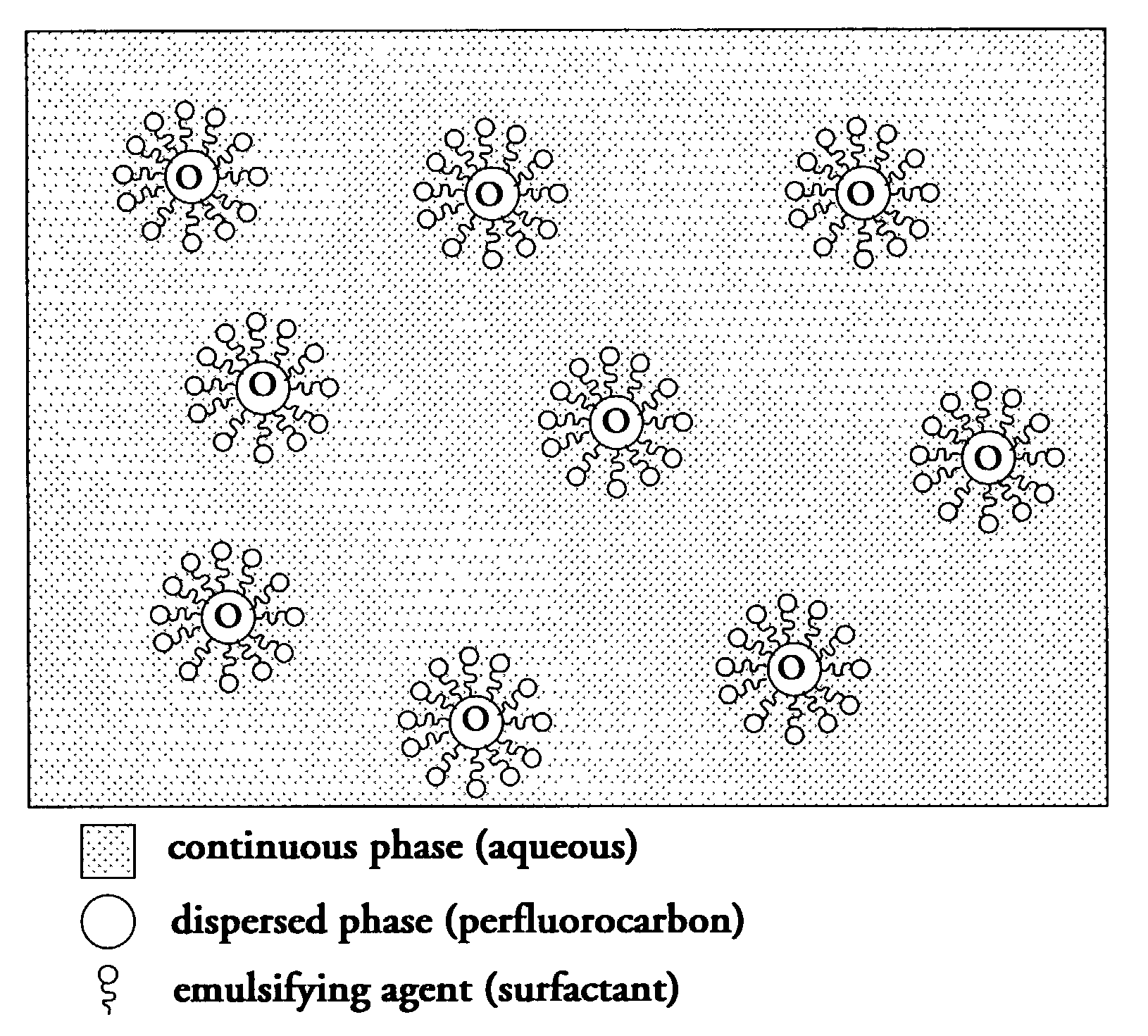
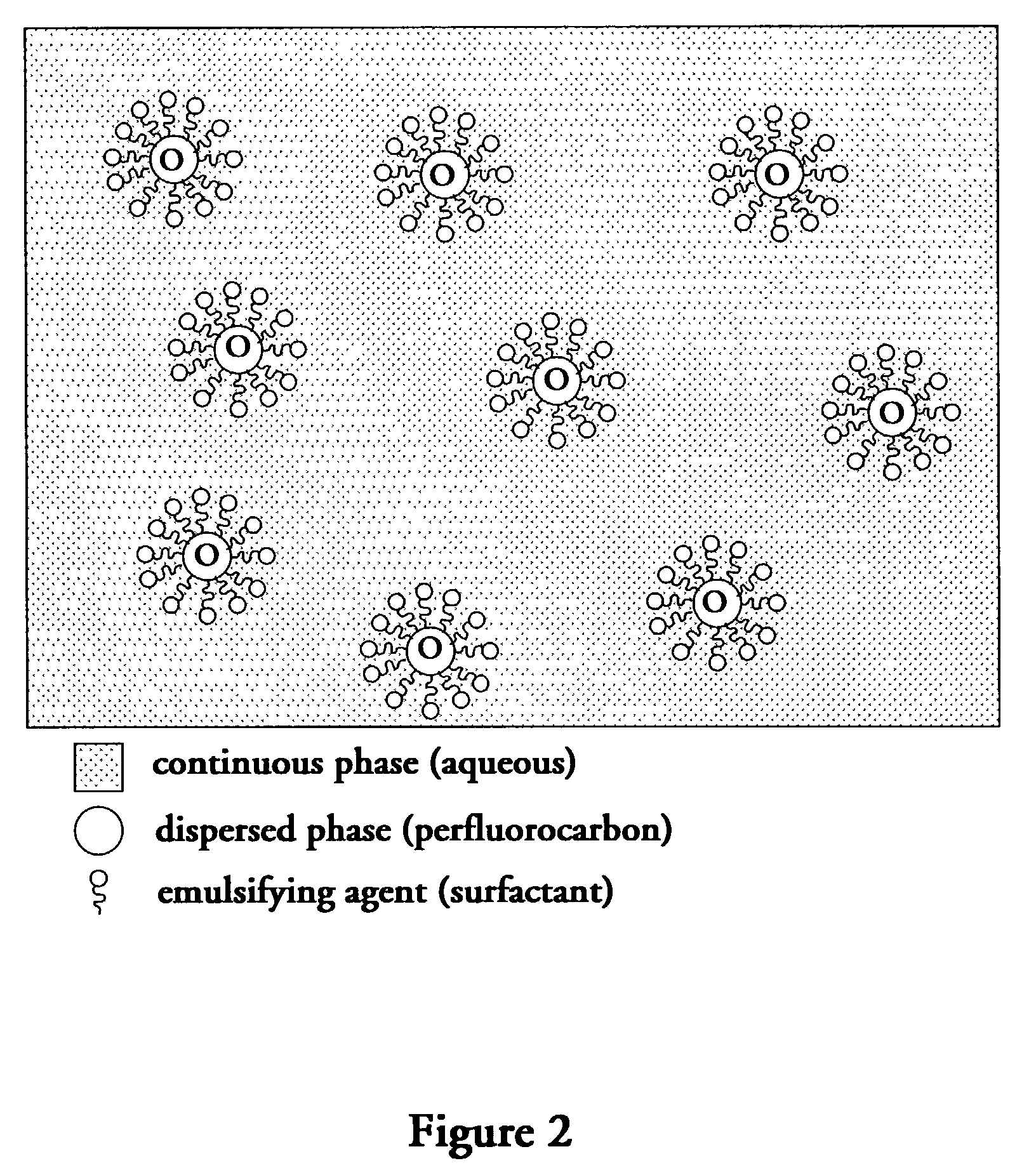
Perfluorocarbon emulsions with non-fluorinated surfactants
InactiveUS7357937B2Emulsion stabilizationSimple and low methodCosmetic preparationsHalogenated hydrocarbon active ingredientsBoiling pointHydrophile
A stable FC emulsion is described. The FC emulsion of the present invention comprises a continuous FC immiscible hydrophilic liquid phase and a dispersed phase comprising FC suspended as droplets within the continuous phase. The emulsion further comprises an emulsifying agent and a stabilizing agent. The stabilizing agent of the present invention reduces the ability of the FC droplets to move within the continuous phase. The present invention also provides a method of making a FC emulsion. The method comprises mixing an FC immiscible hydrophilic liquid and a solid emulsifying agent by agitation at a temperature elevated above the phase transition temperature of the emulsifying agent and below the boiling temperature of the FC immiscible hydrophilic liquid, and adding FC to the mixture of step (a) and agitating at the elevated temperature to disperse droplets of FC in the FC immiscible hydrophilic liquid to form the FC emulsion. The invention also provides another method of making an FC emulsion, which does not require a solid emulsifying agent. The method comprises mixing an FC immiscible hydrophilic liquid and an emulsifying agent to form a first mixture; mixing a stabilizing agent with the first mixture to form a second mixture; and mixing FC with the second mixture to form a third mixture to disperse droplets of FC in the FC immiscible hydrophilic liquid and to form the FC emulsion, wherein the stabilizing agent reduces ability of the droplets to move within a continuous phase of the FC emulsion.
Owner:OXYGEN EMULSION CO LLC
Conductive composition
InactiveCN104981911AExcellent adhesionGood dispersionApparatus for heat treatmentNon-conductive material with dispersed conductive materialParticulatesGlass particle
Disclosed is a conductive composition useful for the preparation of electrically conductive structures on a substrate comprising a plurality of metal particles, a plurality of glass particles and a vehicle comprising at least one cellulose derivative and at least one solid organopolysiloxane resin dissolved in a mutual organic solvent. The solid organopolysiloxane resin acts as adhesion promoter and assists in stably dispersing the metal and glass particles to avoid an agglomeration of such particles without degrading the rheological properties. From such conductive compositions uniform well adherent electrically conductive structures essentially free from defects in the form of cracks, bubbles or coarse particulates can be prepared on dielectric or semiconductor substrates such as silicon wafers in an efficient and cost-saving manner e.g. by screen printing, drying and sintering while inducing only low warping of the substrate. These characteristics render said conductive compositions particularly useful for the fabrication of electrodes of a semiconductor solar cell helping to increase the cell conversion efficiency.
Owner:NUTRITION & BIOSCIENCES USA 1 LLC
Saponin-containing composite type hydrate anti-agglomerant
ActiveCN102925126AAvoid hydrate coalescenceAddress mobile security issuesDrilling compositionWater contentToxicity
The present invention relates to a saponin-containing composite type hydrate anti-agglomerant, which comprises a saponin plant extract and an auxiliary anti-agglomerant. Compared to the existing anti-agglomerant, the saponin-containing composite type hydrate anti-agglomerant of the present invention has the following characteristics that: an initial generation state of a hydrate can be effectively controlled so as to effectively avoid possibly-generated hydrate aggregation when water content is high, such that dynamic control of the hydrate is easily achieved, it is ensured that hydrate crystals exist in an oil-gas-water multiphase mixing transportation pipeline in a small particle form, and accumulation and deposition phenomena can not be generated so as to effectively solve the flow safety assurance problem of the multiphase mixing transportation pipeline; the saponin plant extract in the hydrate anti-agglomerant is a nature plant extract, does not have toxicity, and is easily biodegraded, and the selected raw materials of the auxiliary anti-agglomerant are non-toxic; and the hydrate anti-agglomerant has characteristics of no toxicity, economy, environment protection, good action effect, uniform generated hydrate particle distribution, and the like.
Owner:CHINA UNIV OF PETROLEUM (BEIJING)
PU elastic paint
ActiveCN102229782ASolve the problem of prone to bloomingEasy constructionPolyurea/polyurethane coatingsSolventChemistry
The invention discloses a PU elastic paint, which comprises a main paint, a curing agent and a diluent, wherein the main paint comprises 27 to 35 percent of elastic resin, 0.2 to 1 percent of drier, 1 to 3 percent of elastic powder, 5 to 7 percent of matting agent, 50 to 60 percent of solvent and 1 to 4 percent of organic silicon assistant. The elastic paint disclosed by the invention is easy in construction, the spraying thickness exerts little affect on the luster of the paint film, and the paint film offers a full handfeel and has a soft luster and uniform chroma.
Owner:ZHEJIANG UVCHEM SPECIAL COATINGS CO LTD
Micronized freeze-dried particles
InactiveUS7029700B2Prevents coalesenceMinimize timePowder deliveryPeptide/protein ingredientsSolventChemistry
A process is provided for making dry, micronized particles of an agent, such as a drug. The method includes (a) dissolving a macromolecular material, preferably a polymer, in an effective amount of a solvent, to form a solution; (b) dissolving or dispersing the agent in the solution to form a mixture; (c) freezing the mixture; and (d) drying by vacuum the mixture to form solid particles of the agent dispersed in solid macromolecular material. The micronization in this process occurs directly in a macromolecular matrix and hardening of the particles of agent by solvent removal takes place by lyophilization of the bulk matrix, which stabilizes the drug particles during hardening and prevents coalesence, thereby resulting in smaller final drug particles. The method is particularly preferred for protein agents. The process can be used in conjunction with a standard microencapsulation technique, typically following separation of the agent from the macromolecular matrix. The process yields microparticles having a homogenous size distribution, preferably less than 2 μm, and more preferably less than 1 μm, in size. The microparticles have well defined, predictable properties, which is particularly critical in drug delivery applications.
Owner:BROWN UNIV RES FOUND INC
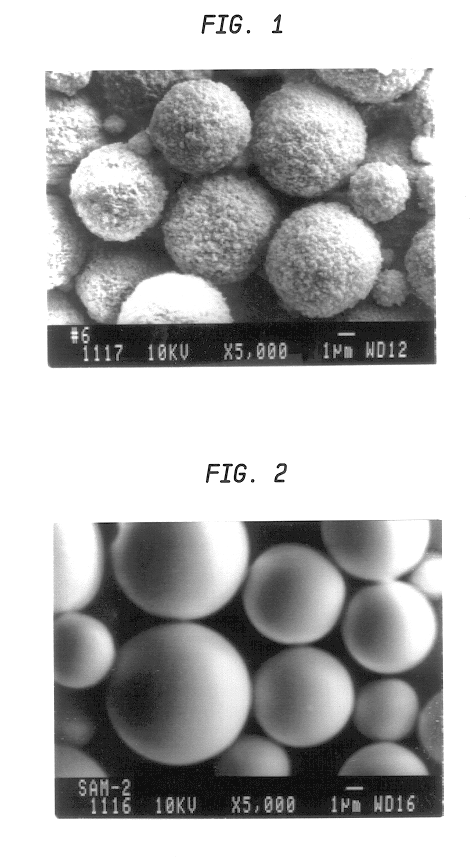
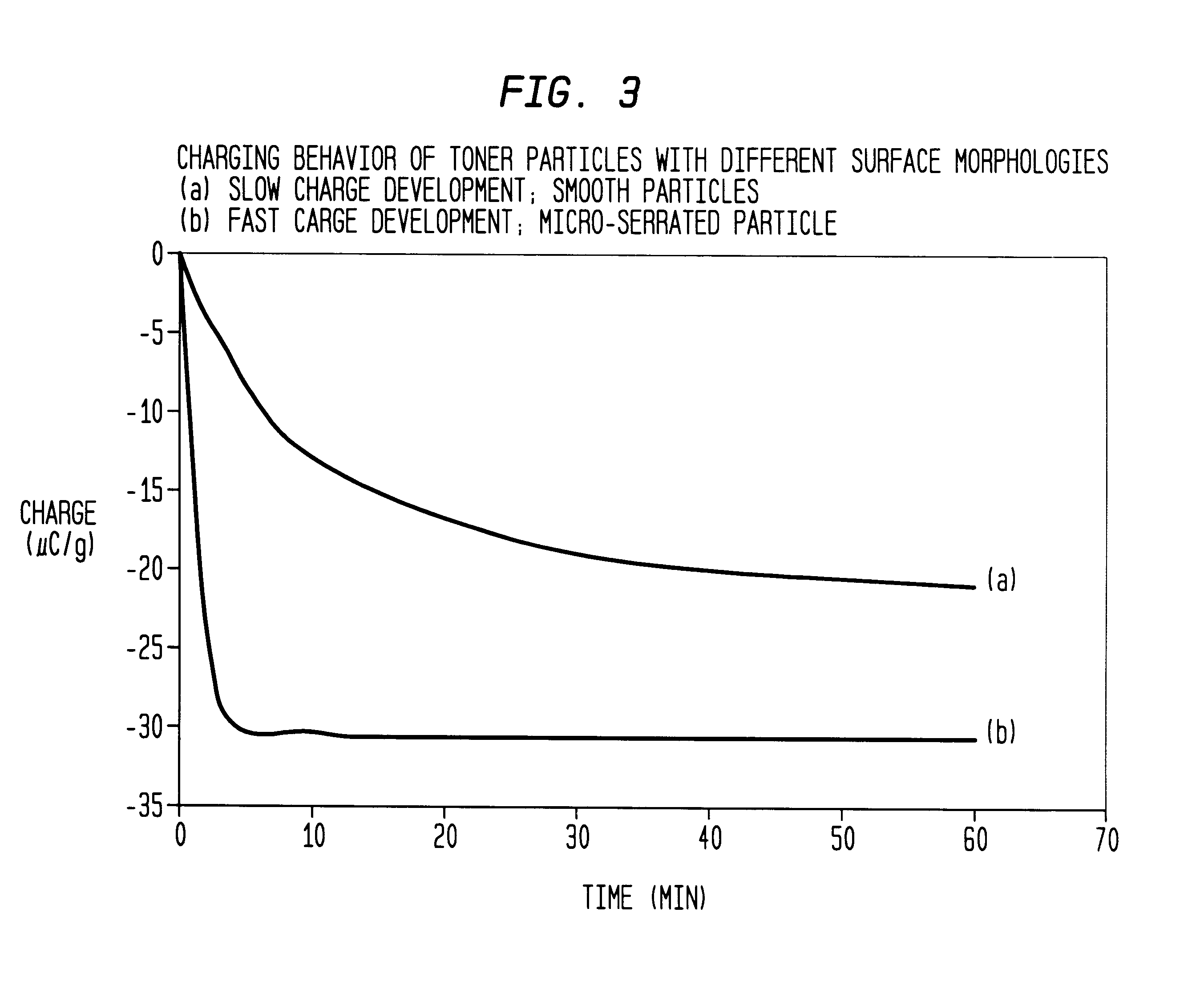
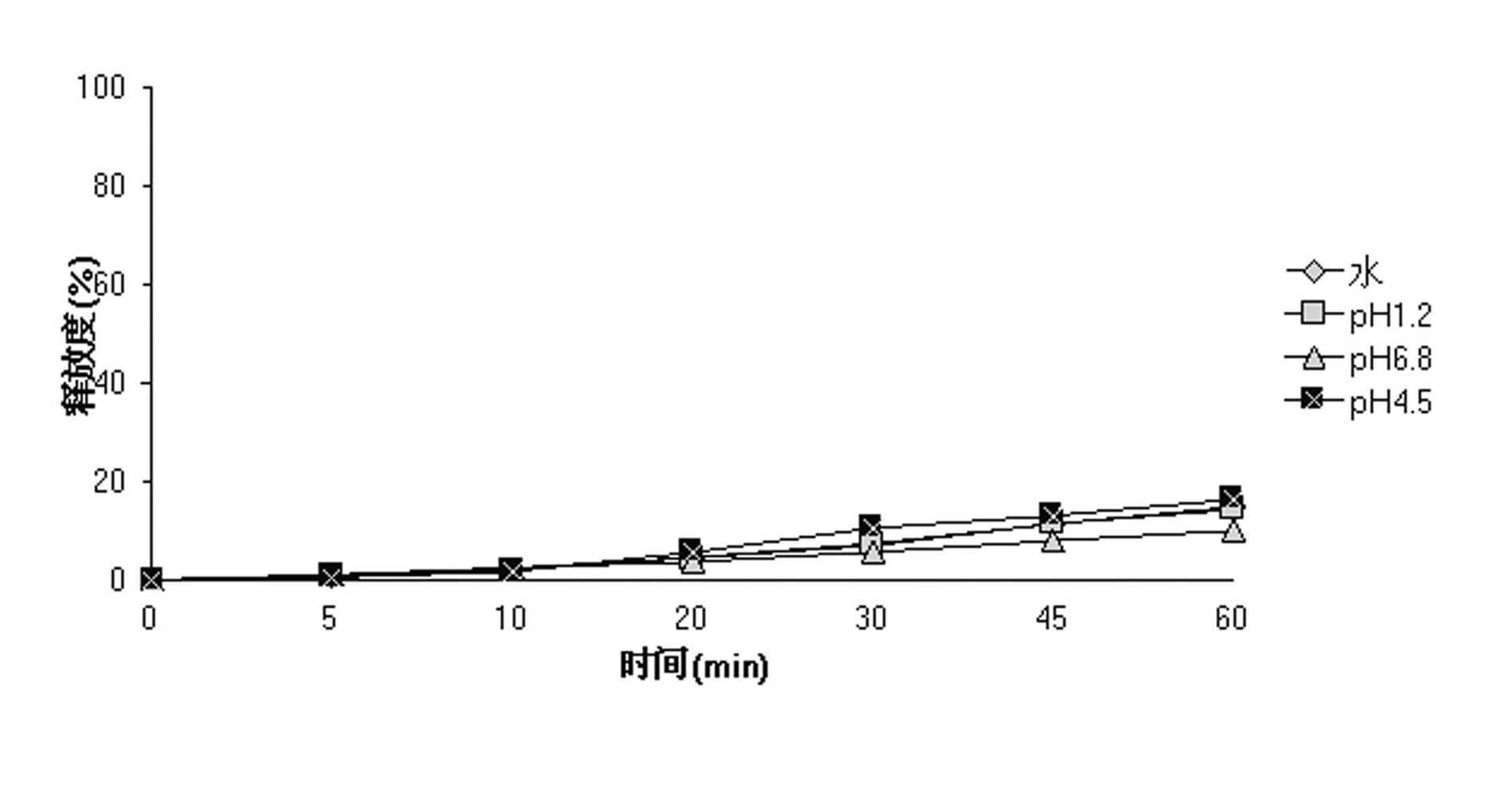
Micro-serrated, dyed color toner particles and method of making same
InactiveUS6544705B2Improves triboelectric charging characteristicIncrease surface areaDevelopersVolume averageRoughness exponent
A color toner composition includes dyed toner resin particles having a volume average diameter in the range of from about 2 microns to about 10 microns with a size distribution span value of less than 1.0. Preferred particles are characterized further by a micro-serrated surface exhibiting a roughness index value larger than about 1.2. The resin toner particles are prepared utilizing a dye-mediating co-solvent which facilitates transfer from a dispersion medium to the resin particles.
Owner:DPI SOLUTIONS INC
Andrographolide ground suspending liquid, preparation method thereof, and application of pharmaceutical preparation
InactiveCN102614133AImprove stabilityDissolution rate is fastAntibacterial agentsOrganic active ingredientsImmediate releaseDrugs preparations
The invention relates to andrographolide ground suspending liquid, a preparation method thereof, and the application of pharmaceutical preparation, which belongs to the field of pharmaceutical preparation. The preparation method comprises the steps of adding andrographolide into hydrophilic accessory solution with certain concentration, and grinding the andrographolide in a basket grinder to prepare suspending liquid with particle sizes smaller than 3000 nm. Liquid layers of pharmaceutical suspending liquid are laminated onto blank pellet cores with certain particle size range, to prepare andrographolide immediate-release pellets. After grinding, by reducing pharmaceutical particle sizes, increasing particle surface areas and improving the wettability of pharmaceutical particles, the dissolution in vitro of the drug is improved, and hydrophilic carriers are adopted to effectively prevent the aggregation of pharmaceutical particles so as to improve the stability of the pharmaceutical preparation. The preparation method is simple and easy for industrialized production, and the dissolving-out speed of the prepared andrographolide immediate-release pellets is high, so that the bioavailability is obviously improved.
Owner:SHENYANG PHARMA UNIVERSITY
Acetylene-hydrochlorinated low-content gold compound catalyst
ActiveCN103191760ADip evenlyImprove conversion ratePhysical/chemical process catalystsPreparation by halogen halide additionAcetyleneAlkali metal
The invention discloses an acetylene-hydrochlorinated low-content gold compound catalyst, particularly a nonmerculic catalyst which is suitable for synthesis of chloroethylene through an acetylene hydrochlorination reaction. The catalyst comprises carrier active carbon, a main catalysis element and auxiliary elements A and B, wherein the content of the carrier active carbon is 10,000 parts, the main catalysis element is thiosulfate of gold with the content of 1-120 parts, the auxiliary element A is thiosulfate of silver with the content of 50-1,000 parts, the auxiliary element B is alkali metal compound and a mixture of the alkali metal compounds with the content of 50-1,000 parts. According to the acetylene-hydrochlorinated low-content gold compound catalyst disclosed by the invention, the content of gold in the prepared gold compound catalyst is low; the cost of noble metal catalyst is remarkably reduced; the prepared gold compound catalyst is a good-activity, strong-stability and high-selectivity novel nonmerculic catalyst; and the acetylene-hydrochlorinated low-content gold compound catalyst has the advantages of simple production process, short production period and environment-friendliness.
Owner:XINJIANG TIANYE GRP +1
Healthy and nutritious low calorie, low fat foodstuffs
InactiveUS20090263555A1Low costStable maintenanceMilk preservationFrozen sweetsGramVolumetric Mass Density
A low calorie, low fat food product of a foodstuff and a stable foam. The foam has a liquid matrix, gas bubbles and a structuring agent that forms a lamellar or vesicle cage structure without generating a gel imparting a rubbery texture. The lamellar / vesicular cage structure entraps a substantial portion of the bubbles and liquid matrix therein in a sufficiently compact structure that substantially prevents drainage of the liquid matrix and coalescence and creaming of the bubbles to maintain stability of the foam even when the foam is subjected to heat shock. The food product contains less than 0.5 grams of fat, and provides a caloric density of less than 200 kcal per each 100 ml serving of gas bubble-free liquid matrix.
Owner:NESTEC SA
Nitrogen and phosphorous doped carbon supported nanoparticle platinum electrocatalyst and method of making
ActiveUS20180272320A1Simple structureIncreased durabilityMembranesSemi-permeable membranesPlatinumPhosphate
A platinum-carbon electrocatalyst material comprising a carbon support having a minimum BET surface area of 1000 m2 / g, a nitrogen content of at least 2.5 weight percent, which is present in the form of pyridine, pyridone or pyrrole, a phosphorous content of at least 3 weight percent, which is present in the form of phosphate and phosphonate, and a plurality of platinum nanoparticles dispersed on the carbon support having a maximum average particle diameter of 1.5 nm.
Owner:TDA RES
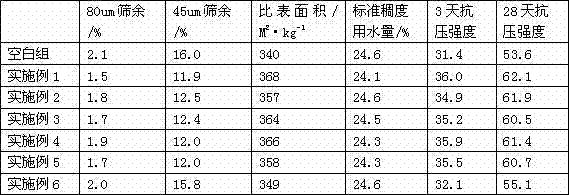
Slag composite activated grinding aids, and preparation method
This invention relates to a method for preparing composite active grinding aid for slag. The composite active grinding aid is composed of: triethanolamine 10-40 wt.%, polycarboxylic acid water reducer 10-40 wt.%, and saturated solution of calcium hydroxide 20-50 wt.%. The method comprises: mixing the raw materials, and activating by magnetization equipment. The composite active grinding aid can improve the fineness and activity of slag, increase grinding efficiency by 10-20%, and increase 3-day strength by 2-3 MPa and 28-day strength by 4-8 MPa. Besides, the composite active grinding aid has such advantages as good grinding effect, low energy consumption and simple production process.
Owner:WUHAN UNIV OF TECH
Cement composite grinding aid
The invention discloses a cement composite grinding aid which is characterized by comprising the following components in parts by weight: 10-30 parts of polyol amine, 10-30 parts of polyol, 5-15 parts of molasses, 5-15 parts of lignosulphonate, 2-6 parts of sodium metasilicate pentahydrate, 3-7 parts of sodium hexametaphosphate and 20-30 parts of water. The cement composite grinding aid disclosed by the invention is stable in raw material source, environmental-friendly, low in toxicity and easy to produce, and ensures that the components achieve preferable synergistic effect by confirming the components and a content range, thus optimizing the efficacy of a powder grinding process and improving the power granule distribution and morphology of cement without adverse effect on cement quality. The cement composite grinding aid disclosed by the invention has the advantages of preferably increasing the yield increase and enhancing the cement quality and has the effects of saving the electricity consumption, improving the powder efficiency and the cement strength, reducing the free calcium oxide contained in the cement and enhancing the early strength and later strength of the cement.
Owner:湖州华仑助剂科技有限公司
Thermolysis of organic waste in a ball furnace
ActiveCN1863606APrevent coalescenceSolid waste disposalTransportation and packagingThermal energySteel ball
The method is characterised in that the thermal energy necessary for thermolysis of the waste carried out in the absence of air is provided by a heating mass, comprising steel balls (9), running in the furnace (1) co-currently with the waste. The method may be applied to household waste, sewage work residues, hospital wastes, wastes of risk to the agro-food industry and in a general manner to all wastes containing organic matter be it of urban, agricultural or industrial origin.
Owner:FINAXO ENVIRONNEMENT
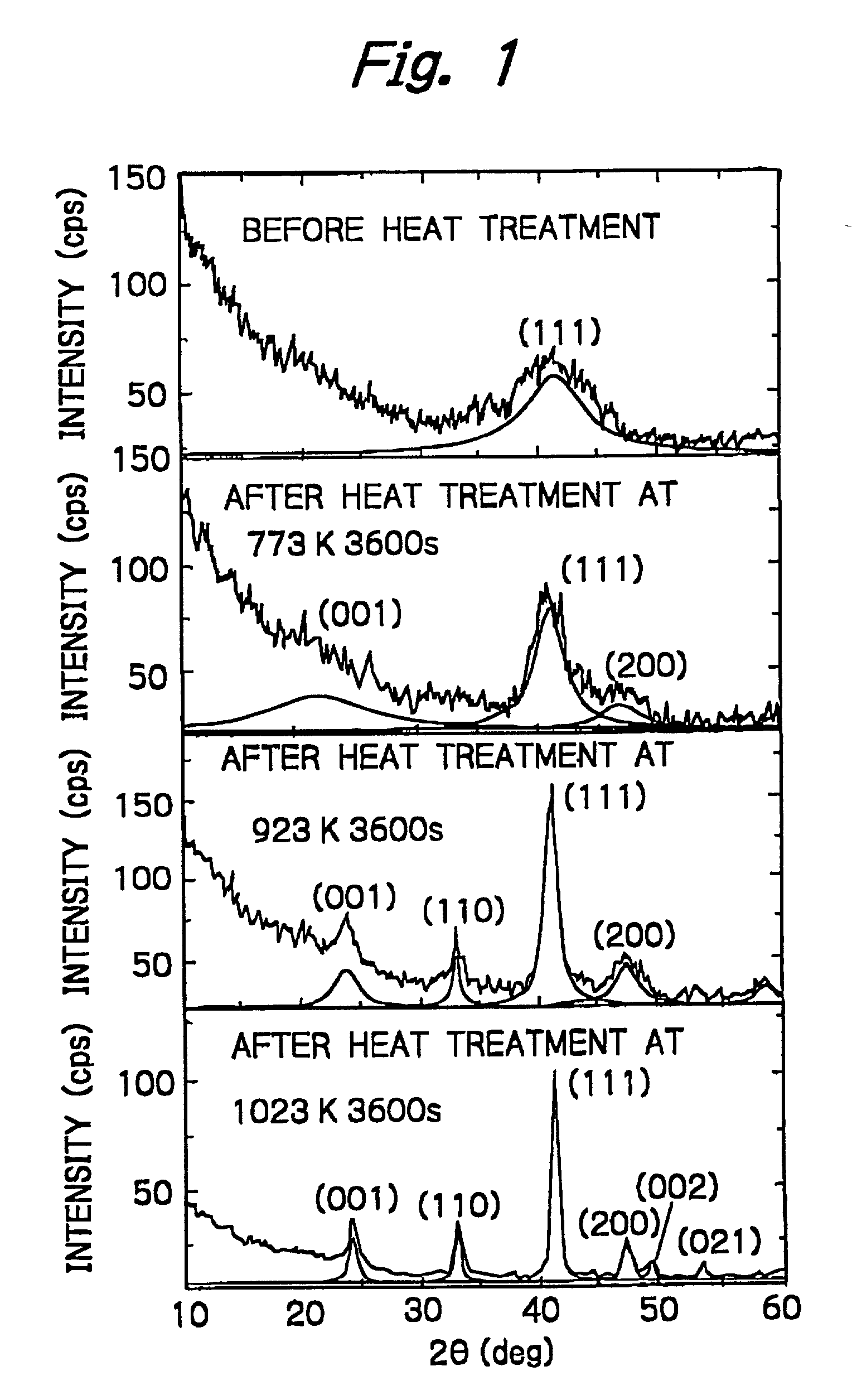
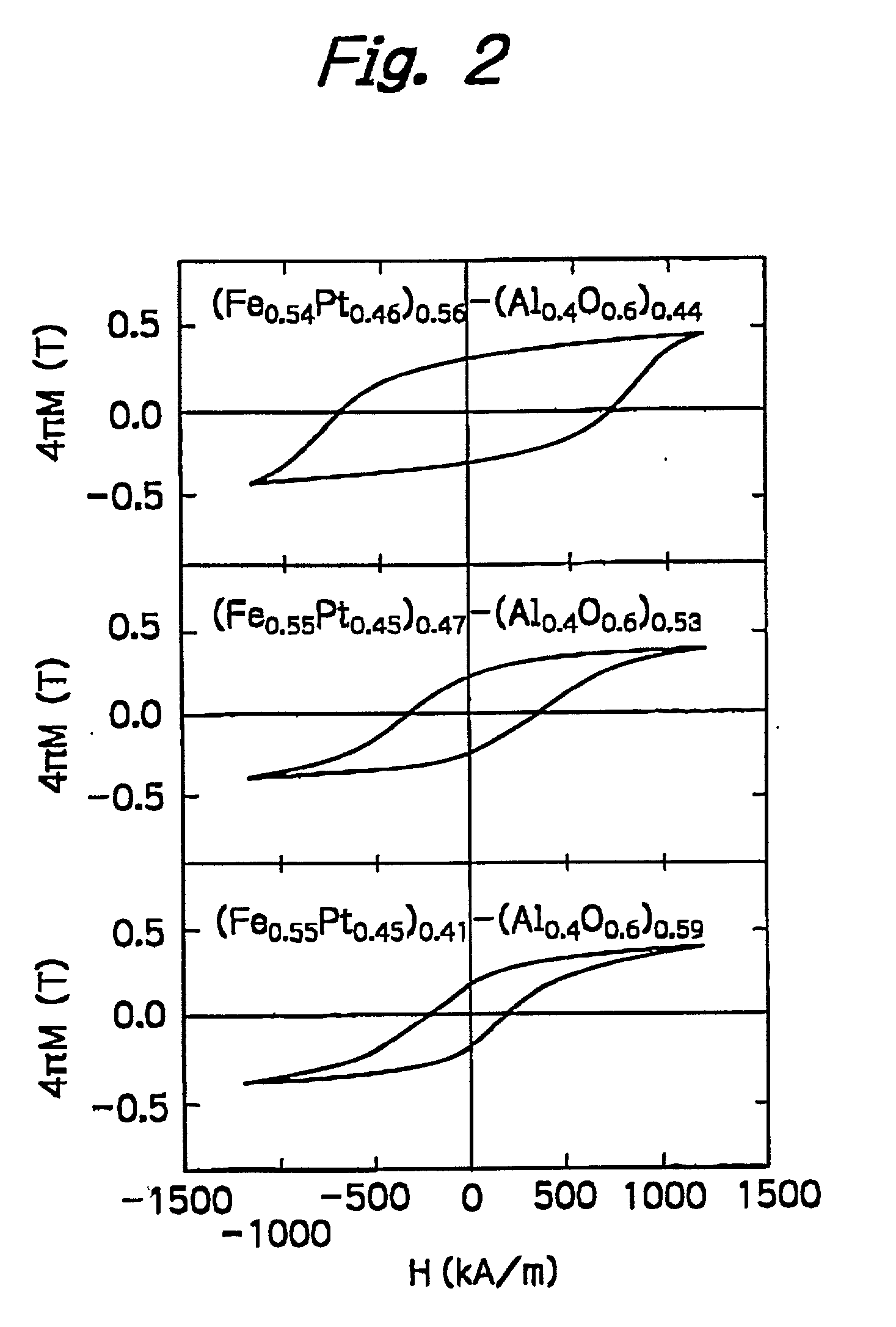
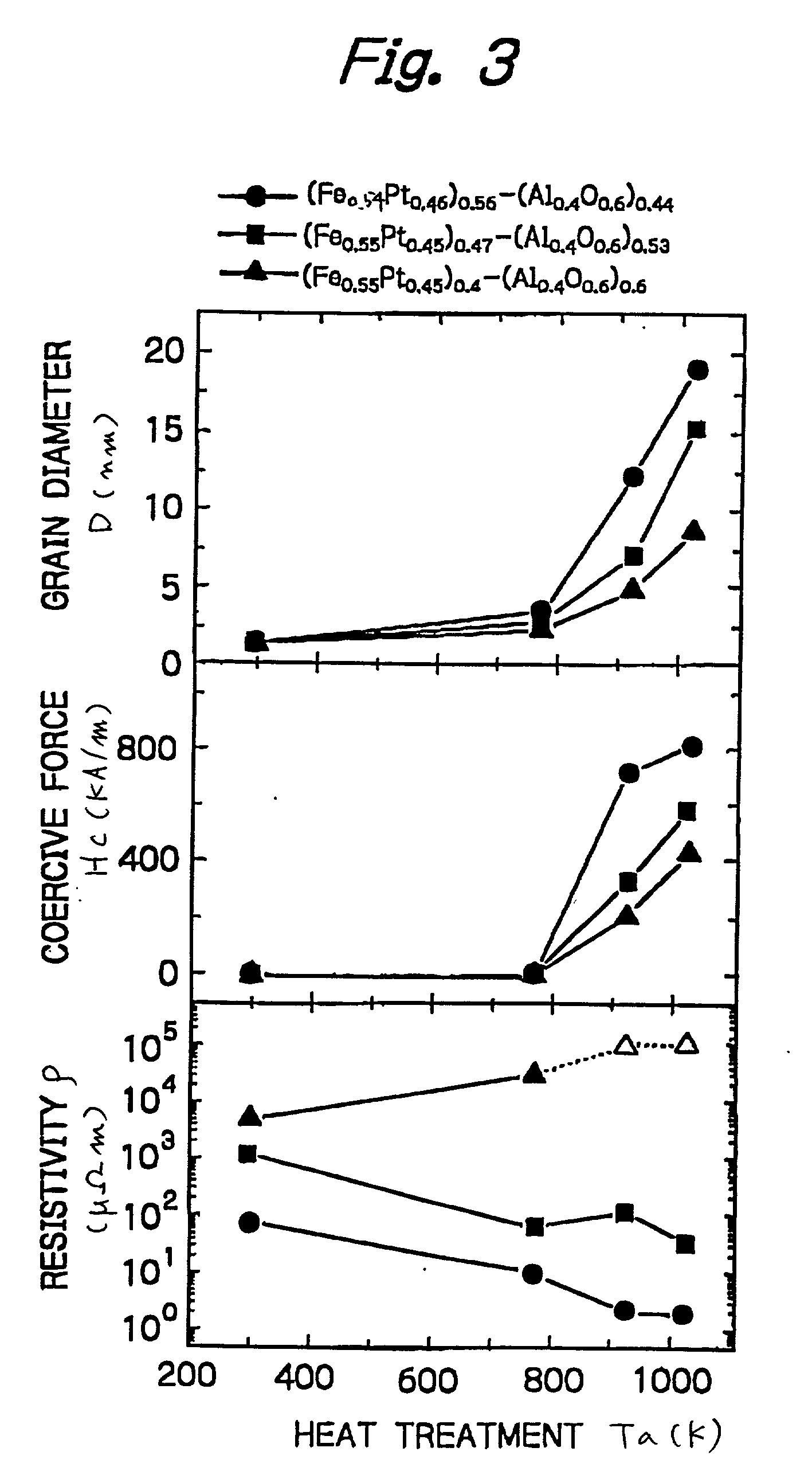
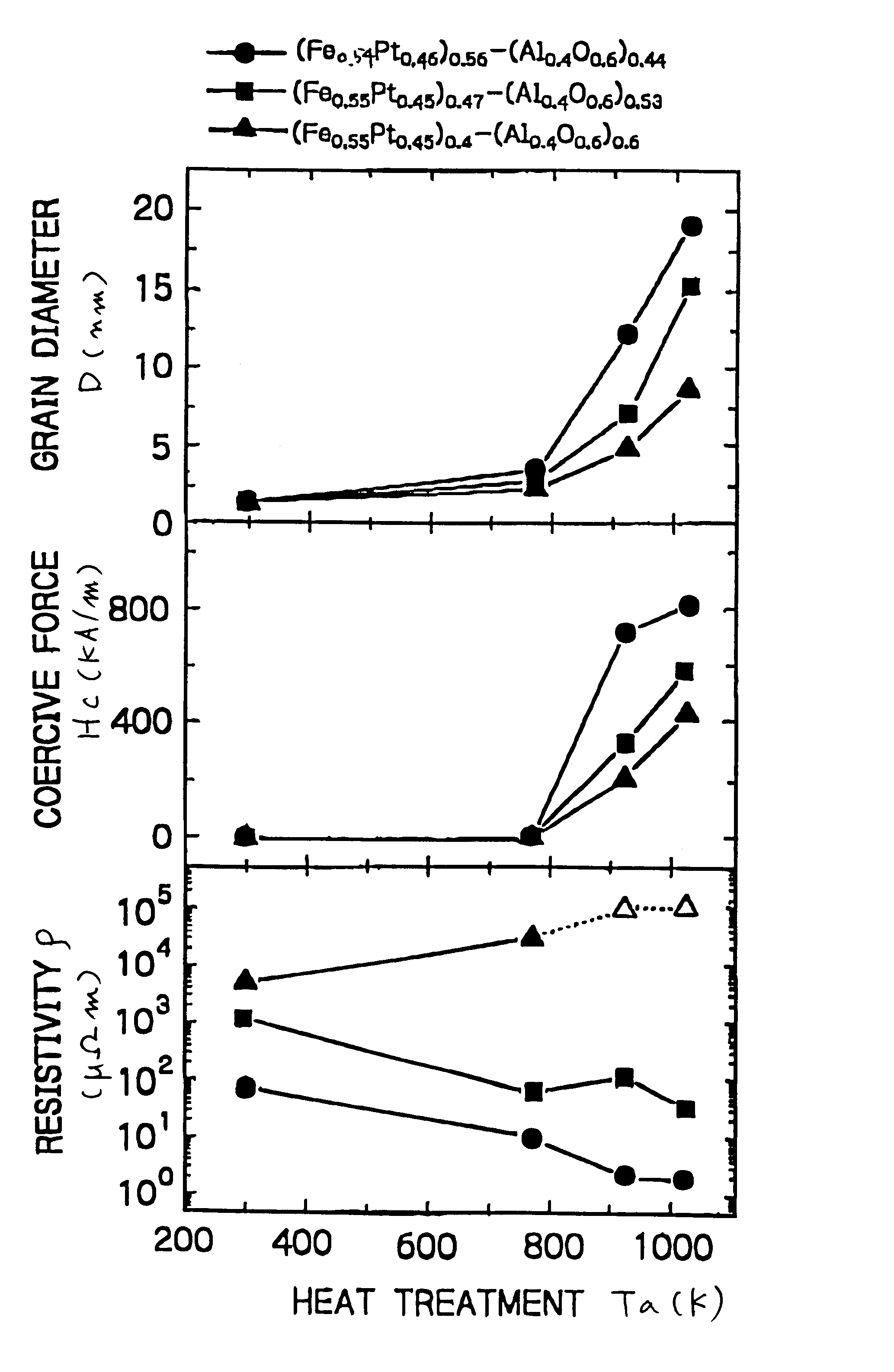
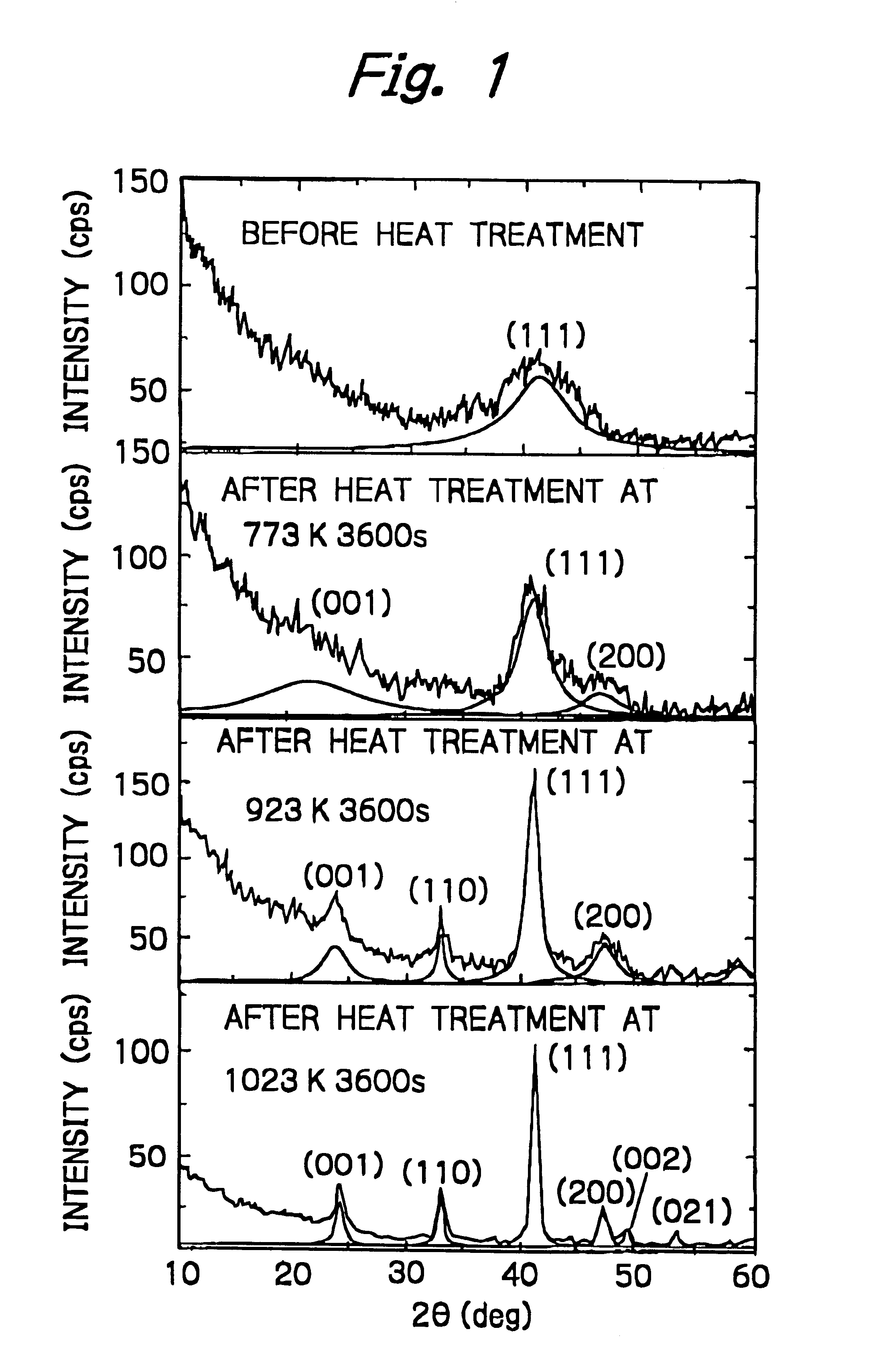
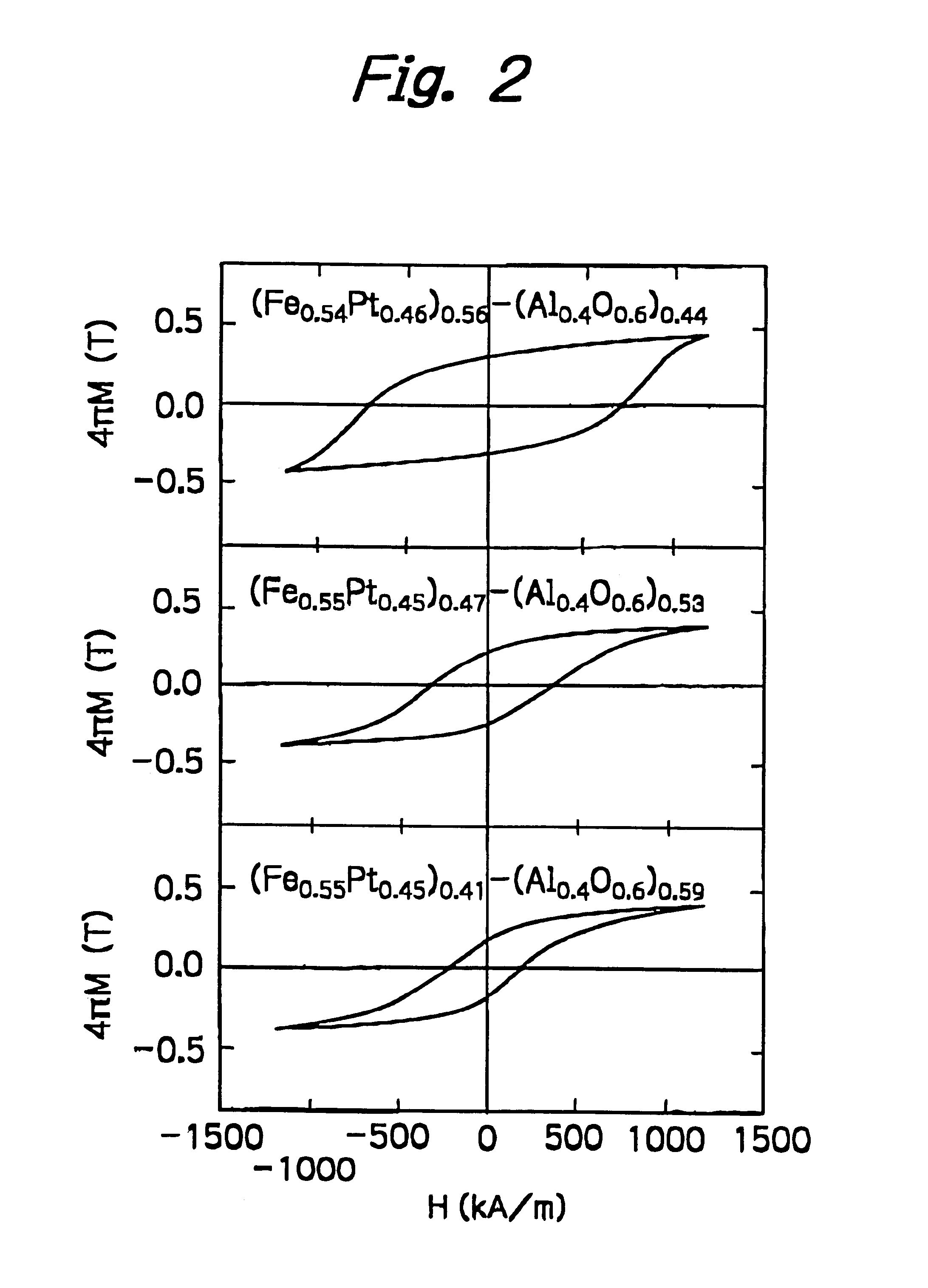
Nanogranular thin film and magnetic recording media
InactiveUS20010036563A1Improve thermal stabilityReduce noiseNanomagnetismMaterials with cobaltNanometreOptoelectronics
A nanogranular thin film consisting of nonmagnetic matrix and ferromagnetic fine particles in nano scale is improved to enhance the thermal stability and the S / N ratio. The ferromagnetic fine particles consist of (FeaCo1-a)1-xPtx, (0.3<=x<=0.7, 0.1<=x<=1), (FeaCo1-a)1-xPdx, (0.3<=x<=0.7, 0.1<=x<=1) or (FeaCo1-a)1-x(PtbPd1-b)x, (0.3<=x<=0.7, 0.1<=x<=1, and 0<b<1).
Owner:FOUND THE RES INST FOR ELECRTRIC & MAGNETIC MATERIALS THE
Cu@mSiO2 core-shell nano catalyst for preparing hydrogen from ammonia borane and hydrazine borane by hydrolysis and preparation method of catalyst
ActiveCN103949254AImprove performanceEasy to useHydrogen productionMetal/metal-oxides/metal-hydroxide catalystsNano catalystHydrazine compound
The invention provides a Cu@mSiO2 core-shell nano catalyst for preparing hydrogen from ammonia borane and hydrazine borane by hydrolysis and a preparation method of the catalyst. The catalyst is prepared by an inverted micelles method in a kettle. A core is formed by non-noble metal copper (Cu), and a core-shell nanostructure of a shell is formed by mesoporous silica (mSiO2). The catalyst is analyzed by a TEM image, the core is formed by metal Cu nano particles, and the shell is of the core-shell nanostructure of mesoporous SiO2. The catalyst has the characteristics of being small in core metal particles, clear in core-shell structure and morphology, uniform in size, large in specific surface area, excellent in activity and cycle performance, and the like, and the shell is made from mesoporous SiO2.
Owner:JIANGXI NORMAL UNIV
Crystallization method of cefadroxil monohydrate and crystals
ActiveCN102134250AInhibit the rate of crystallizationPrevent coalescenceOrganic chemistryGranularityCEFADROXIL MONOHYDRATE
The invention relates to a method for producing crystals of cefadroxil monohydrate, comprising the following steps: adding an N, N-dimethyl formamide solvent compound of cefadroxil to a mixed solvent of N, N-dimethyl formamide and water, wherein the mass ratio of the cefadroxil solvent compound to the mixed solvent is 1:1-1:2, stirring to obtain a suspending liquid, and keeping the temperature at15-30 DGE C and the pH value of the solution at 6.0-7.5 during feeding; after the cefadroxil solvent compound is totally changed into the cefadroxil monohydrate, reducing the pH value of the suspending liquid to 4.5-5.0 within 1-20 minutes, keeping the temperature at 10-18 DEGC, and stirring for crystal cultivation for 10-90 minutes; and filtering, washing and drying after crystal cultivation is finished to obtain a product, the square platy crystal-form cefadroxil monohydrate. The square platy crystal-form cefadroxil monohydrate obtained by the method has uniform particle size distribution, does not generate coalescence, and is applicable to industrial production.
Owner:TIANJIN UNIV
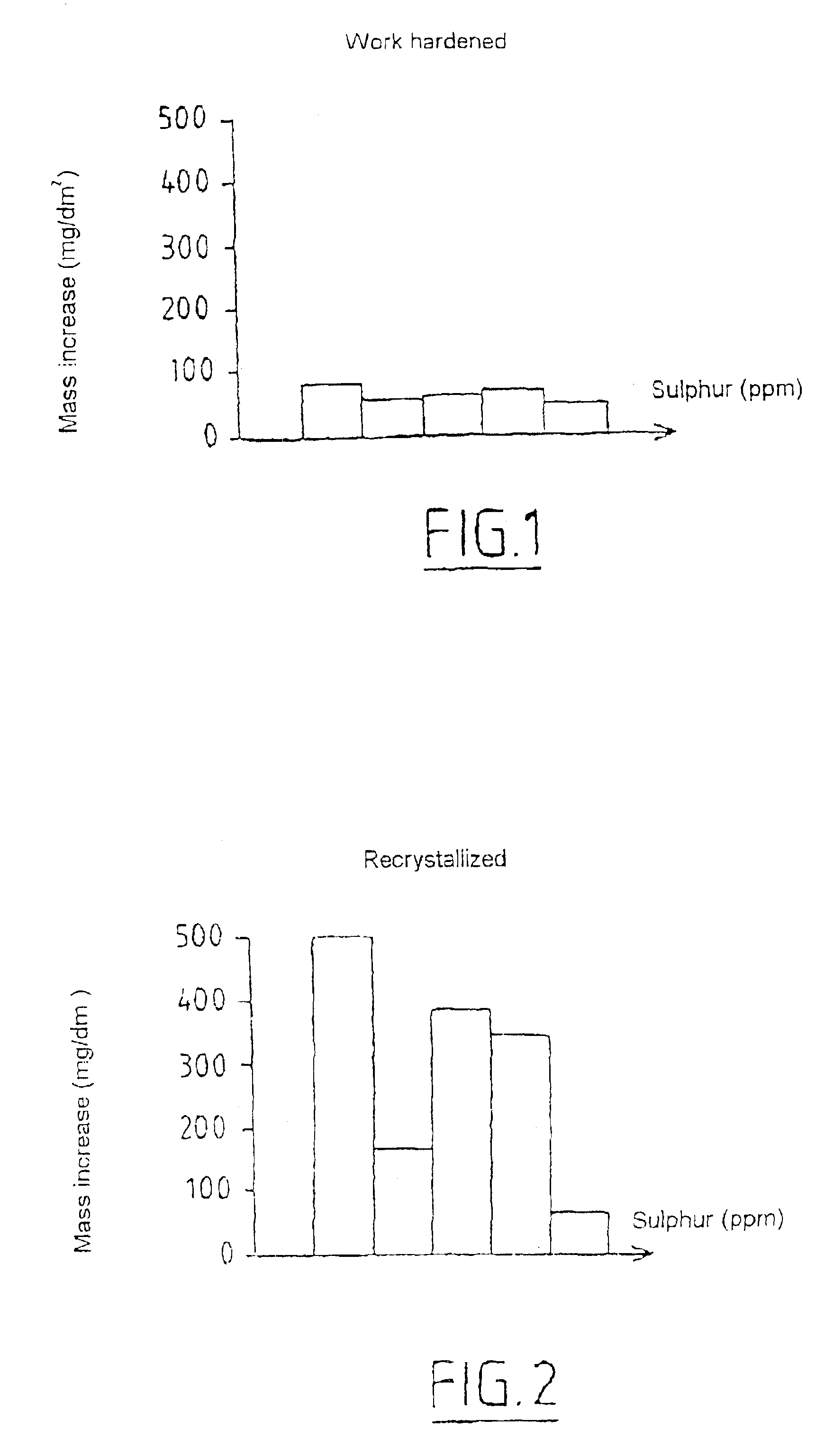
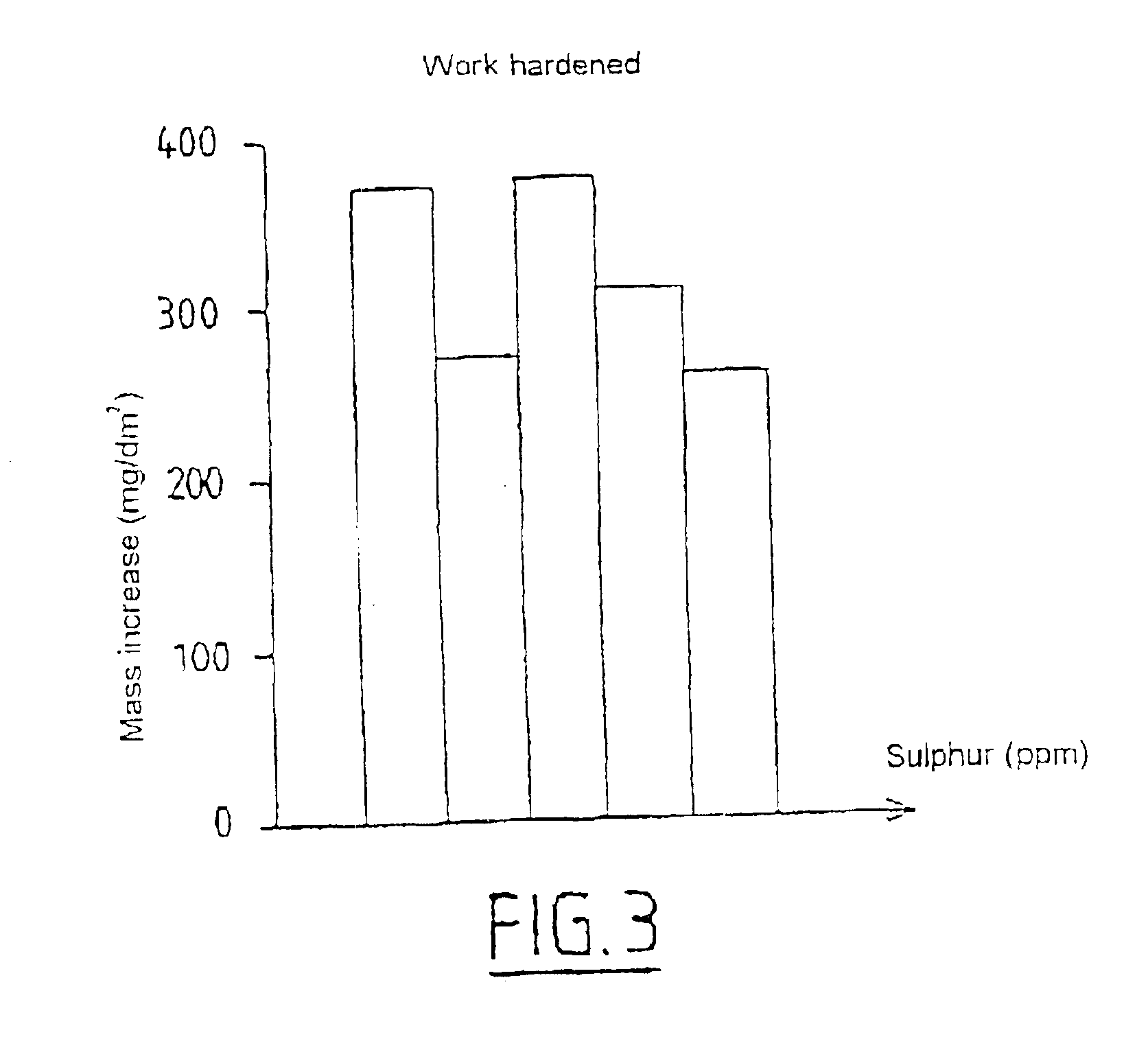
Zirconium alloy highly resistant to corrosion and to sun burst by water and water vapor and method for thermomechanical transformation of the alloy
InactiveUS6884304B1Accelerated corrosionIncrease hydriding resistanceOptical rangefindersFuel elementsWater vaporNiobium
The alloy contains, by weight, at least 95% zirconium and from 0.01 to 0.1% sulphur and, optionally, at least one element from the group consisting of the elements tin, iron, chromium, hafnium, niobium, nickel, oxygen and vanadium, the balance of the alloy consisting of inevitable impurities. The sulphur is present in the alloy in the dissolved state, thereby improving the creep strength and in the form of uniformly distributed fine precipitates, thereby improving the corrosion and hydriding resistance. The alloy may be heated by a solution annealing treatment in the β phase followed by a quench or by a soak at a temperature below 950° C. in order to transform it into the α or α+β phase.
Owner:CO EUROPENNE DU ZIRCONIUM CEZUS
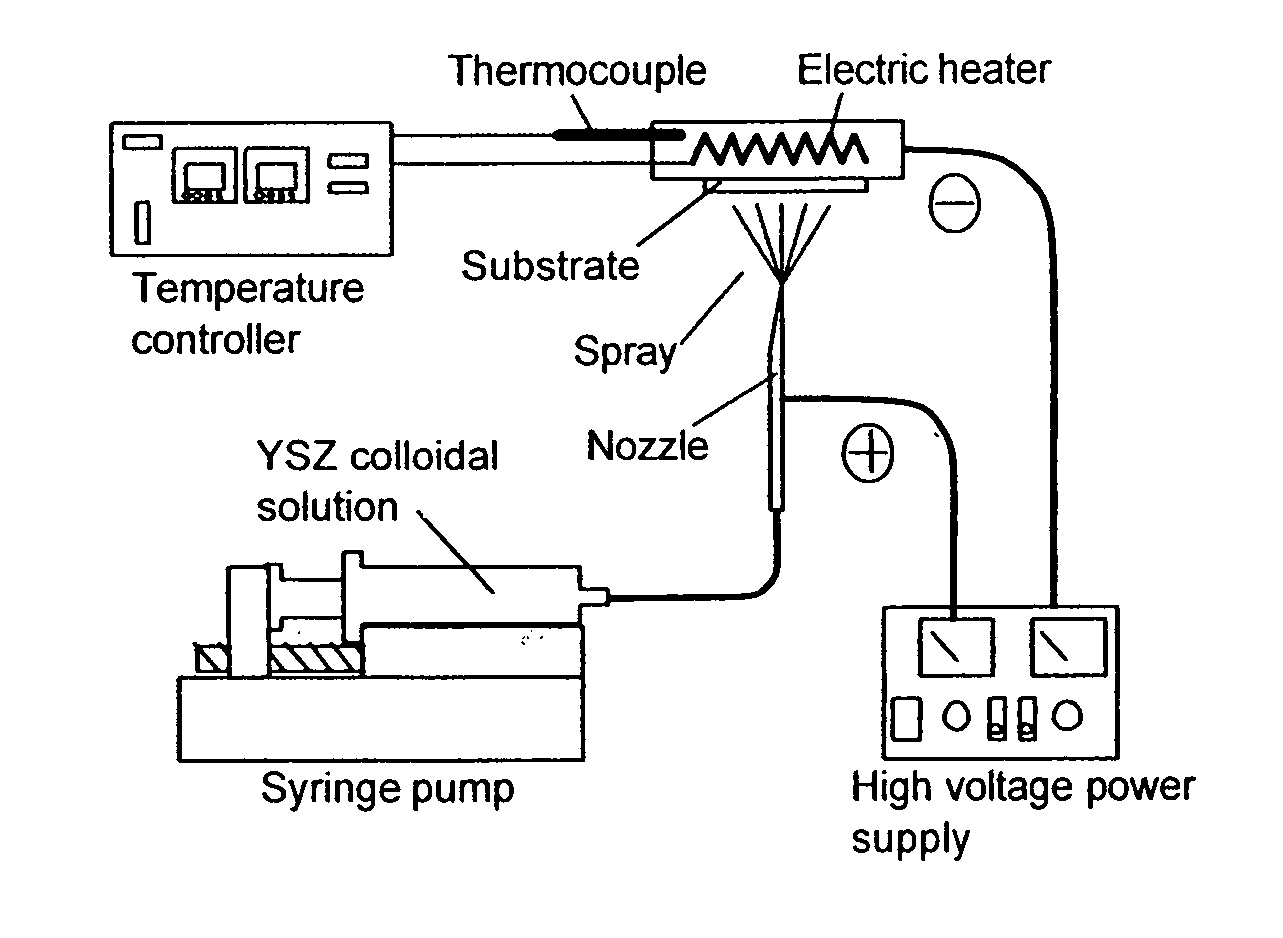
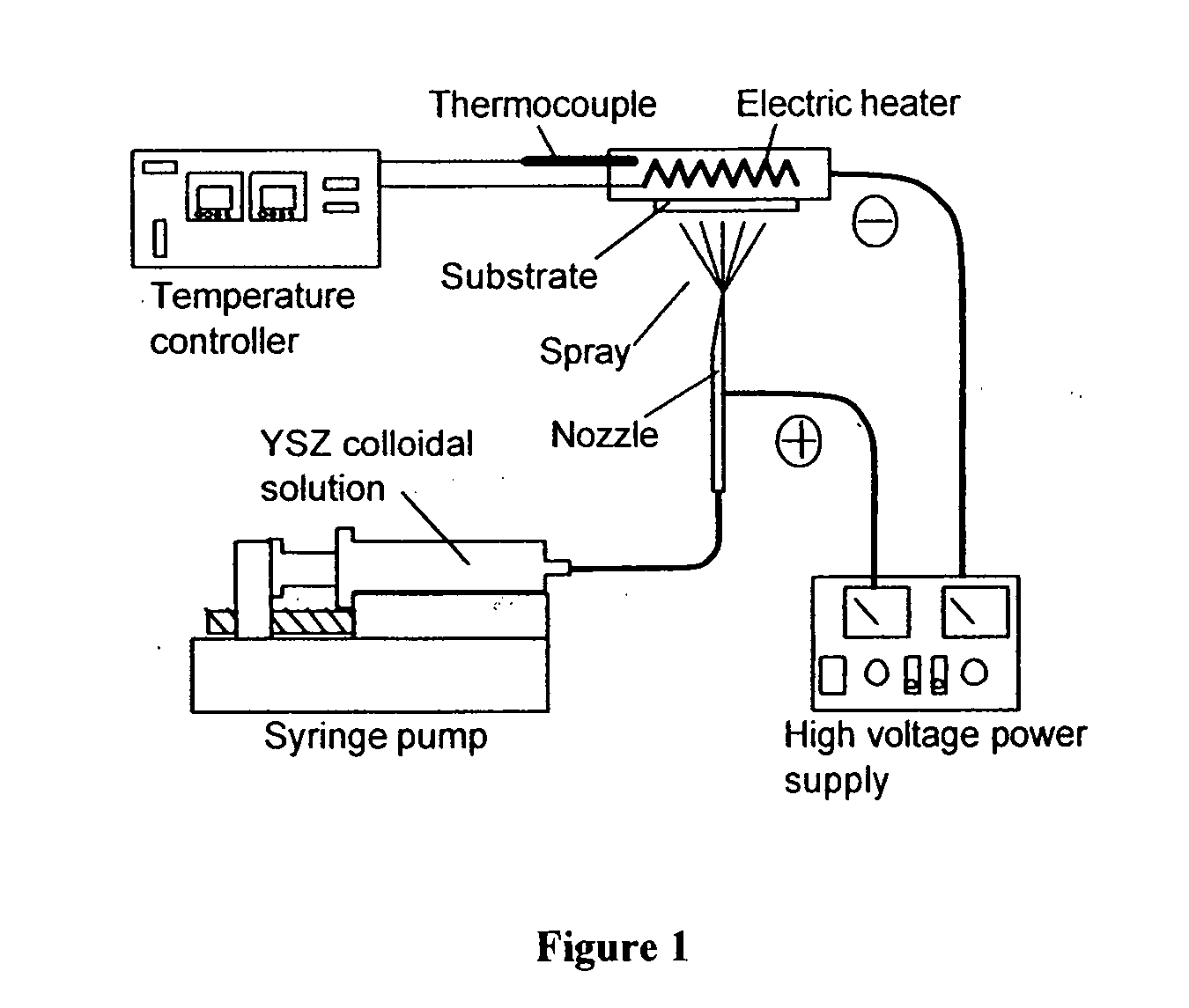
Method and apparatus for electrostatic spray deposition for a solid oxide fuel cell
InactiveUS20080029026A1Easy to set upLarge film growth rateBurnersLiquid surface applicatorsThin layerSpray nozzle
A method and apparatus for electrostatic spray deposition (ESD) for fabricating a thin-layer yttria-stabilized zirconia (YSZ) electrolyte on a solid oxide fuel cell (SOFC) anode substrate constructed of nickel-YSZ cermet. By reducing the thickness of the electrolyte, and thereby reducing the cell internal IR drop, an intermediate temperature SOFC (ITSOFC) can operate at 600-800° C. A collar positioned at a distance from a discharge end of a spray nozzle enhances a spray pattern of a precursor including the electrolyte material and thus provides a very thin electrolyte layer.
Owner:ILLINOIS INSTITUTE OF TECHNOLOGY
Efficient and non-corrosive hydrate inhibitor
ActiveCN101608111AReduce dosageSimplify subsequent processingDrilling compositionPetroleumCalcium nitrate
The invention provides a high-efficient non-corrosive hydrate inhibitor, relating to the technical field of oil-gas hydrate. The hydrate inhibitor is a mixture of an anti-agglomerant and a urea or a calcium nitrate and is used at the pressure of 1.5 to 25 MPa and at the temperature of minus 20 DEG C to 25 DEG C. The hydrate inhibitor overcomes the defects of the prior art, reduces the nucleary, the growth and the coalescence speed of hydrate after being filled in petroleum fluid in production or conveying by mixing at low concentration and has the characteristics of wide application, low cost and no corrosion to pipelines.
Owner:SOUTH CHINA UNIV OF TECH
Nanogranular thin film and magnetic recording media
InactiveUS6623857B2Improve thermal stabilityReduce noiseNanomagnetismMaterials with cobaltNanoparticleOptoelectronics
A nanogranular thin film consisting of nonmagnetic matrix and ferromagnetic fine particles in nano scale is improved to enhance the thermal stability and the S / N ratio. The ferromagnetic fine particles consist of (FeaCo1-a)1-xPtx, (0.3<=x<=0.7, 0.1<=a<=1), (FeaCo1-a)1-xPdx, (0.3<=x<=0.7, 0.1<=a<=1) or (FeaCo1-a) 1-x(PtbPd1-b)x, (0.3<=x<=0.7, 0.1<=a<=1, and 0<b<1).
Owner:FOUND THE RES INST FOR ELECRTRIC & MAGNETIC MATERIALS THE
Amphiphilic fluorine-containing gradient copolymer, and preparation method and application thereof
ActiveCN104927011AEasy to prepareSimple processSemi-permeable membranesWater/sewage treatment bu osmosis/dialysisNon solventUltrafiltration
The invention discloses a preparation method of an amphiphilic fluorine-containing gradient copolymer, and the method is used in oil-water separation ultrafiltration membrane antifouling modification. First, with a reversible addition fragmentation chain transfer (RAFT) controlled free radical polymerization method, with a semi-continuous feeding manner, an amphiphilic fluorine-containing gradient copolymer is prepared; the prepared amphiphilic fluorine-containing gradient copolymer is adopted as a modifying agent, and is used for modifying the ultrafiltration membrane with a blending modification method, wherein the membrane is prepared with a classic non-solvent induced phase transformation method. The surface of the membrane modified with the amphiphilic fluorine-containing gradient copolymer has an amphiphilic hierarchical structure with the coexistence of hydrophilic and hydrophobic regions. The amphiphilic fluorine-containing gradient copolymer preparation method is simple; and the modified membrane preparation process has the advantages of easy operation and mild conditions. The modified membrane has good oil-water separation performance and excellent antifouling effect, and can be used in water treatment and especially the separation purification of oil-containing wastewater.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com