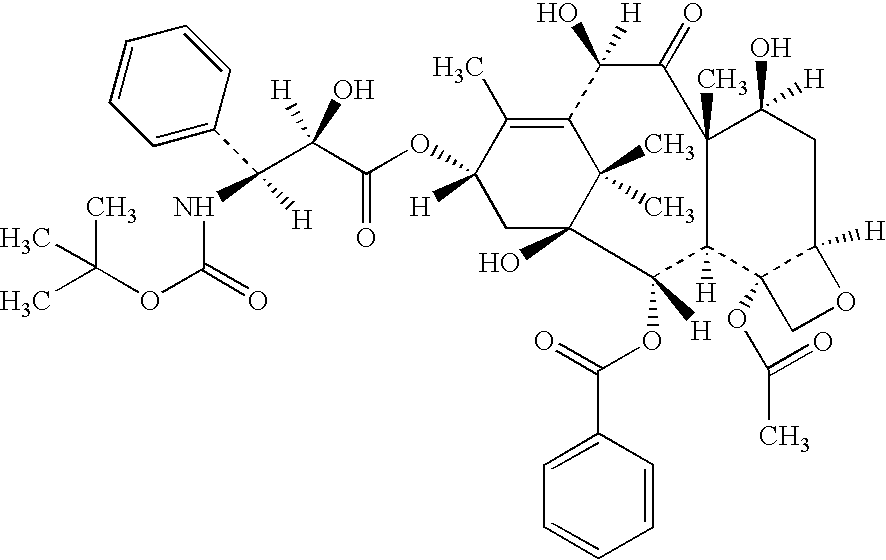
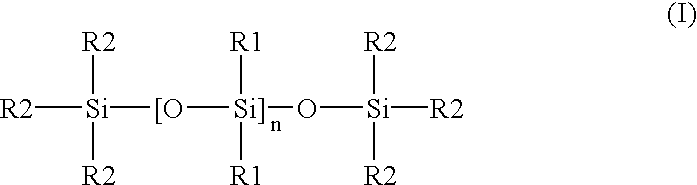
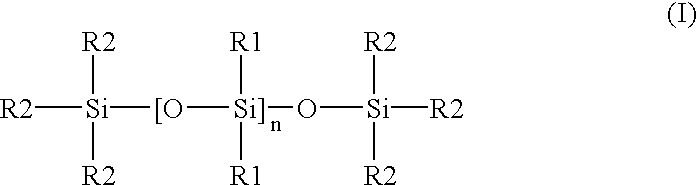
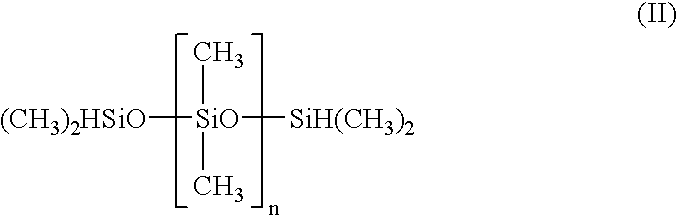Patents
Literature
3881 results about "Oil in water" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions and methods for in vitro sorting of molecular and cellular libraries
InactiveUS20070077572A1Emulsion stabilizationWide potentialPeptide librariesMicrobiological testing/measurementCell biologyIn vitro system
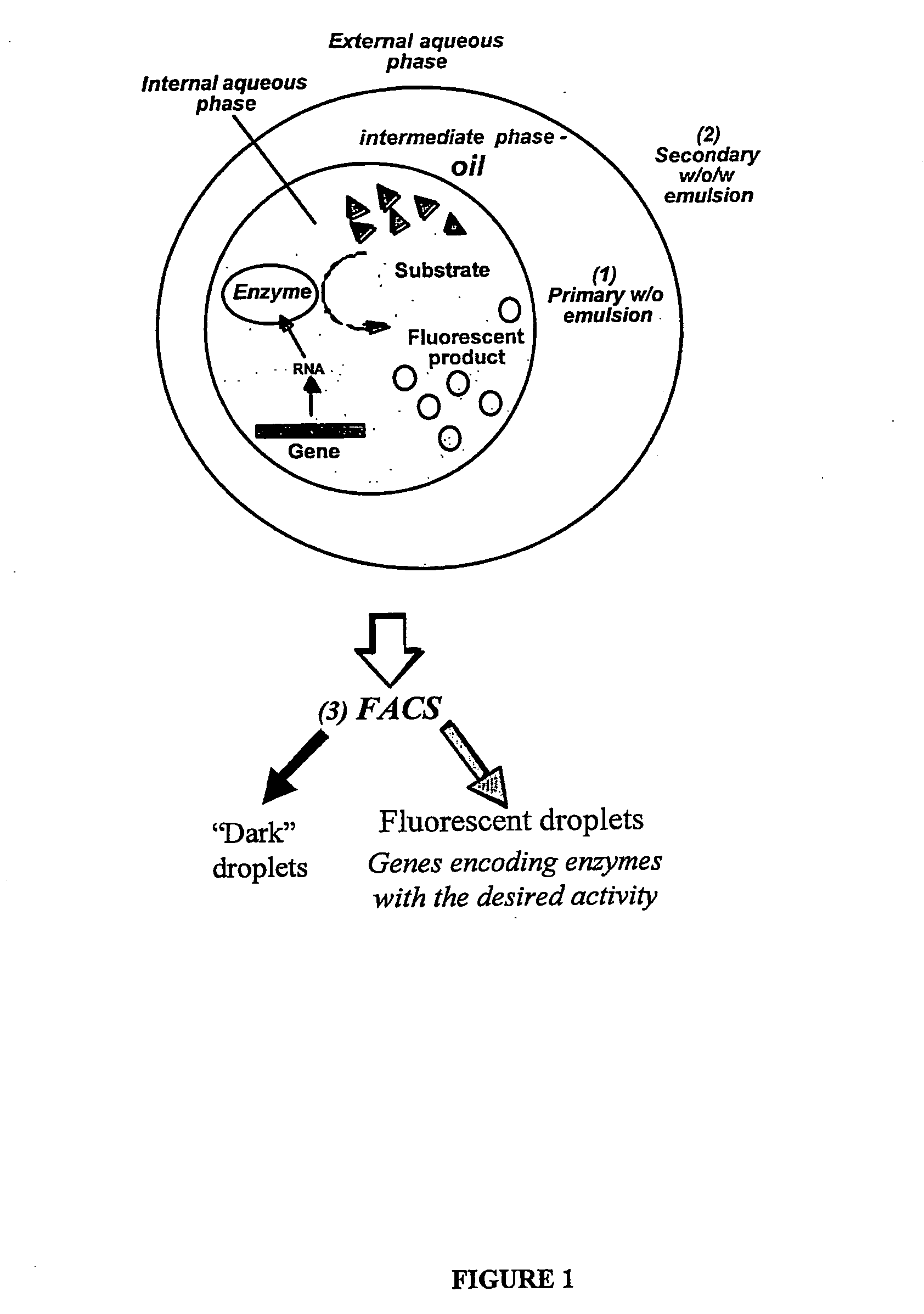
The present invention provides an in vitro system for compartmentalization of molecular or cellular libraries and provides methods for selection and isolation of desired molecules or cells from the libraries. The library includes a plurality of distinct molecules or cells encapsulated within a water-in-oil-in-water emulsion. The emulsion includes a continuous external aqueous phase and a discontinuous dispersion of water-in-oil droplets. The internal aqueous phase of a plurality of such droplets comprises a specific molecule or cell that is within the plurality of distinct molecules or cells of the library.
Owner:MEDICAL RESEARCH COUNCIL +2
Vaccine formulations
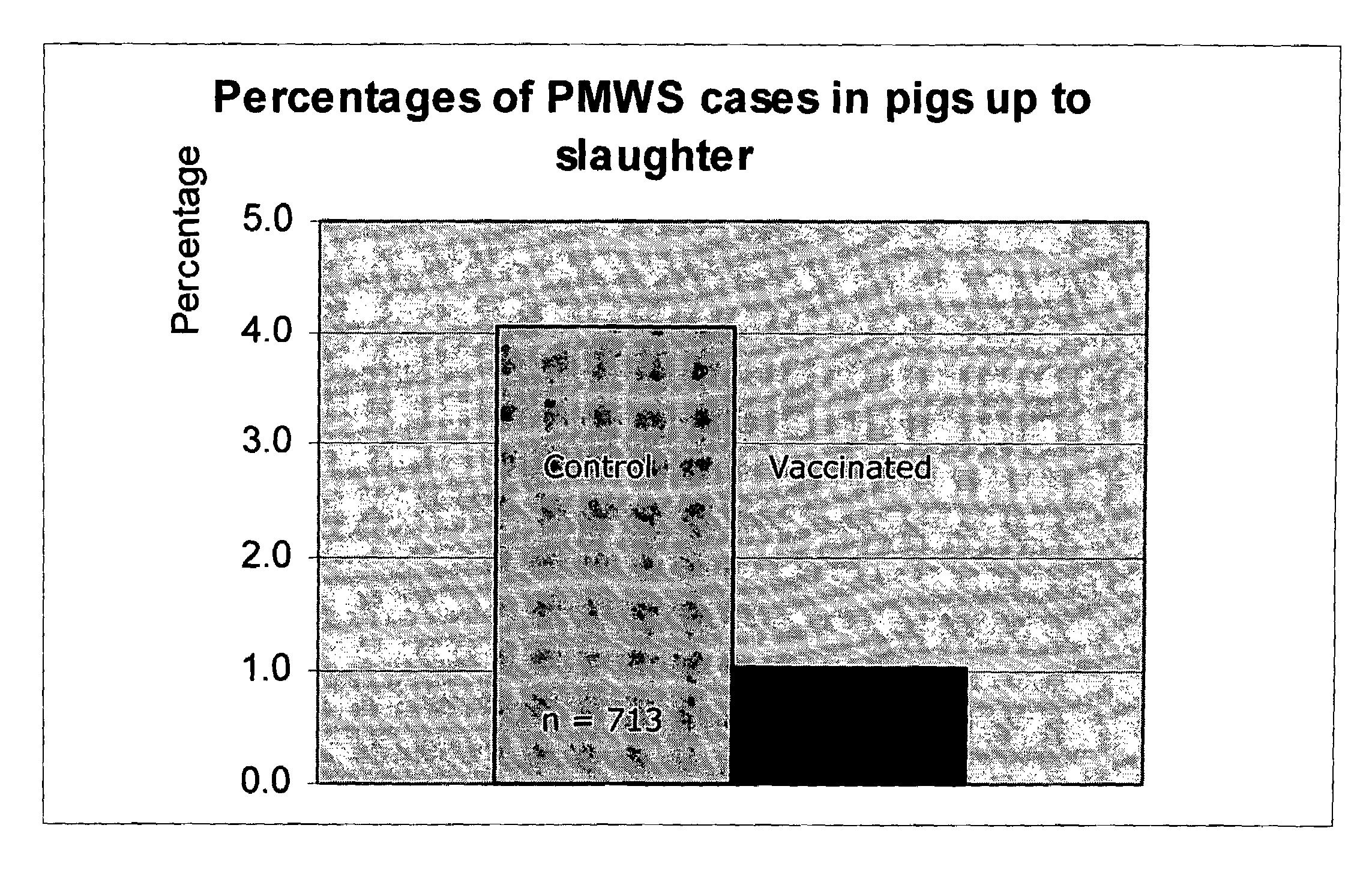
ActiveUS7371395B2Improve stabilityStable and safe and easily administrableSsRNA viruses negative-senseAntibacterial agentsPlasmidBacilli
Owner:MERIAL INC
Crystalline hydroxy waxes as oil in water stabilizers for skin cleansing liquid composition
The present invention relates to a stress stable lathering skin cleansing liquid composition comprising by weight parts of the liquid composition: (a) from about 0.5 parts to 10 parts of a stabilizer; for example trihydroxystearin; (b) from about 1 part to about 80 parts of lipid skin moisturizing agent; (c) from about 1 part to about 30 parts of surfactant having a combined CMC equilibrium surface tension value of from 15 to 50; (d) water; wherein said stress stable lathering skin cleansing liquid composition has a Lipid Deposition Value (LDV) of from about 5 to about 100 and wherein said composition is stable for at least two weeks at 100 F.
Owner:THE PROCTER & GAMBLE COMPANY
Water-based ink for ink-jet recording
A water-based ink for inkjet printing comprising a water dispersion of vinyl polymer particles prepared by containing a pigment in a vinyl polymer prepared by copolymerizing a monomer mixture comprising (a) a salt-forming group-containing monomer, (b) a macromer, and (c) a monomer copolymerizable with the salt-forming group-containing monomer and the macromer; and a process for preparing a water-based ink for inkjet printing comprising a water dispersion of vinyl polymer particles prepared by containing a pigment in a vinyl polymer, comprising dissolving in an organic solvent a vinyl polymer prepared by copolymerizing a monomer mixture comprising (a) a salt-forming group-containing monomer, (b) a macromer, and (c) a monomer copolymerizable with the salt-forming group-containing monomer and the macromer; adding a pigment to the resulting solution; pre-kneading the mixture; thereafter adding a neutralizing agent and water and kneading the mixture, to give an oil-in-water dispersion; and distilling off the organic solvent from the resulting kneaded product.
Owner:KAO CORP
Topical compositions and methods for treating pain
InactiveUS6638981B2Avoid painComposition is stableBiocideNervous disorderNR1 NMDA receptorPreventing pain
Topical compositions and methods for treating pain. The invention provides oil-in-water emulsions comprising an antidepressant; an NMDA-receptor antagonists; a lipophilic component; water; and a surfactant. The compositions induce a local-anesthetic effect when topically administered to intact skin thereby treating or preventing pain, for example, neuropathic pain.
Owner:EPICEPT CORP
Preparation and characterization of formulations in a high throughput mode
ActiveUS20050058574A1Improve throughputFormula flexibleLibrary tagsFlow propertiesAnalysis dataAdditive ingredient
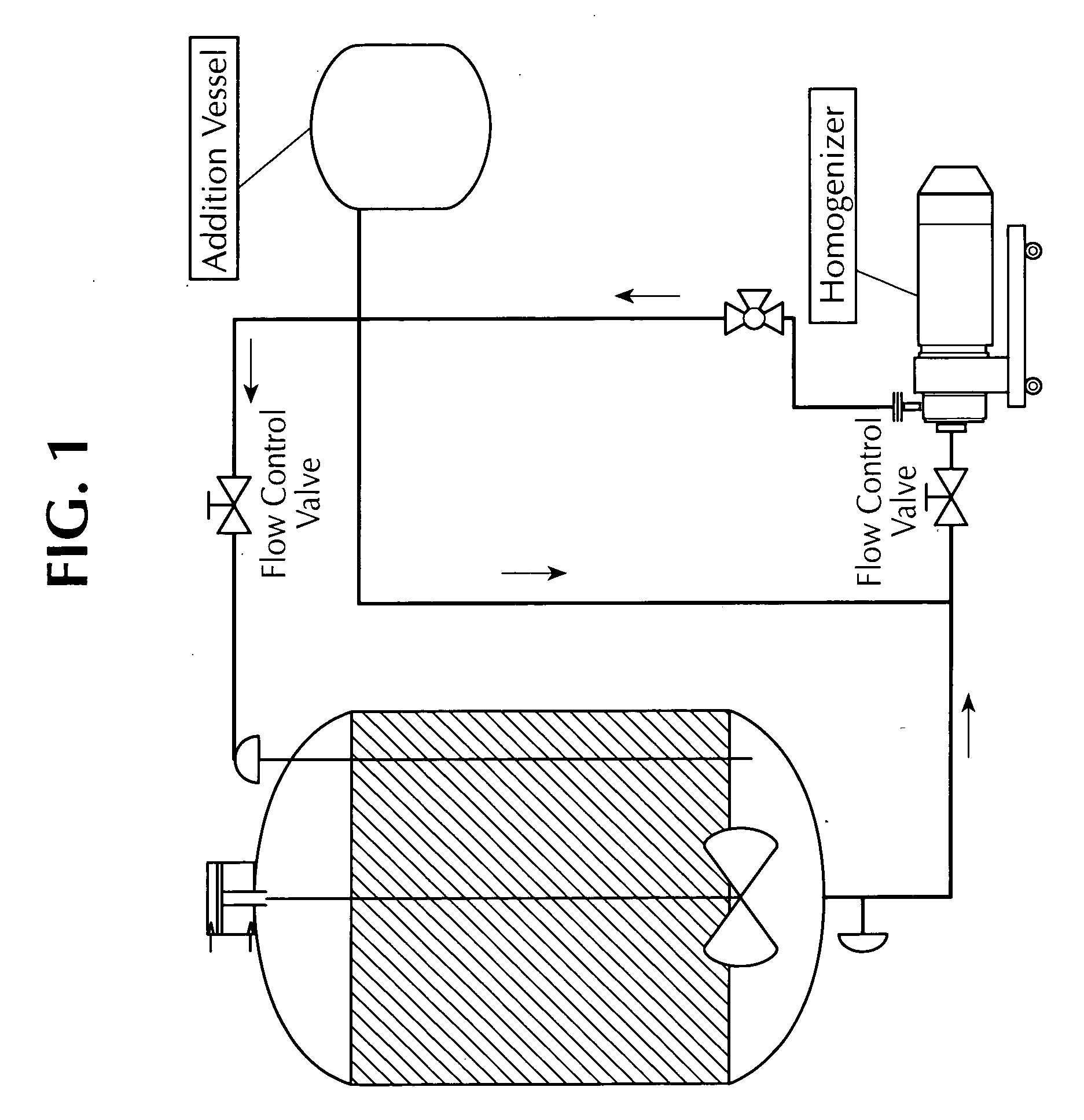
The invention is an automated robotic system for the production and testing of formulations at a very high throughput. It is an integrated system of hardware and software capable of preparing and evaluating hundreds of emulsions per day. The system can formulate aqueous solutions (SL), oil in water emulsions (EW), suspo-emulsions (SE), micro capsule suspensions (CS), micro-emulsions (ME), and suspension concentrates (SC) at the 1 ml to 25 ml scale. The system can process emulsions rapidly in an automated way and enable very flexible formulation recipes to be introduced. The system allows chemists to generate experimental samples of varying recipe and method to be conducted in parallel with projected throughput of up to 1200 formulations processed and characterized per day. Materials and consumables can be distributed from storage storage systems to the work stations where dispensing of ingredients in various states can be performed, including solids, liquids, gels, pastes, suspensions and waxes. The emulsions formed can be characterized using methods including phase diagnosis, turbidity analysis, viscosity and particle sizing using automated test equipment. An integrated module can also perform Tank Mix Compatibility testing in high throughput mode. The modular system allows future processes and tests to be added, either to a station, or as a new station. The software capability includes tracking of processes from start to finish and the integration of analytical data with the as-designed and as-formulated experimental results.
Owner:SYNGENTA LTD
Foam containing unique oil globules
InactiveUS20060233721A1Insufficient hydration of skinCosmetic preparationsAerosol deliveryActive agentNose
The present invention provides a foamable composition for administration to the skin, body surface, body cavity or mucosal surface, e.g., the mucosa of the nose, mouth, eye, ear, respiratory system, vagina or rectum. The foamable oil in water emulsion composition includes: an oil globule system, selected from the group consisting of oil bodies; and sub-micron oil globules, about 0.1% to about 5% by weight of an agent, selected from the group consisting of a surface-active agent, having an HLB value between 9 and 16; and a polymeric agent, and a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition, water and optional ingredients are added to complete the total mass to 100%. Upon release from an aerosol container, the foamable composition forms and expanded foam suitable for topical administration.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Microemulsion process and composition
ActiveUS20060057168A1Quality improvementCosmetic preparationsOrganic active ingredientsVegetable oilSilanes
There is provided a process for the preparation of an oil in water (O / W) microemulsion or sub-micron emulsion composition for dermal delivery of at least one pharmaceutically active ingredient, the method including the steps of a) Admixing a first part including at least one of the group consisting of animal, mineral or vegetable oils, silanes, siloxanes, esters, fatty acids, fats, halogen compounds or alkoxylated alcohols; and one or more lipophilic surfactants, and a second part including water and at least one hydrophilic surfactant to achieve homogeneity, b) heating the mix of step a) to a phase assembly temperature in the range of 40-99° C., preferably 45-95° C., more preferably 65-85° C. with continuous mixing to obtain a microemulsion or sub-micron emulsion, c) allowing said microemulsion or sub-micron emulsion to cool, and d) adding a third part to said microemulsion or sub-micron emulsion at a temperature between 2° C. and said phase assembly temperature, said third part if necessary being premixed and heated until the components are dissolved and including at least one component selected from the group consisting of non-surfactant amphiphilic type compound, surfactant and water with the proviso that when the third part includes water it also includes a non-surfactant amphiphilic type compound and / or surfactant. The phase assembly temperature can be determined visually by the achievement of translucence in the composition or by measures such as conductivity which peaks and then is maintained at a plateau whilst phase assembly occurs. It has been found that whilst if a non-surfactant amphiphilic type compound such as the polyol is added together with the second part as would conventionally be the case, a microemulsion or sub-micron emulsion is not formed, by adding the so called third part, phase assembly occurs at a lower temperature than would be expected and moreover, this phase appears to assist in maintaining the microemulsion or sub-micron emulsion characteristics of the formulation during storage at normal temperatures.
Owner:STIEFEL WEST COAST
Vitamin formulation
A pharmaceutical aerosol foam composition, comprising: an effective amount of a pharmaceutically active ingredient, wherein said pharmaceutically active ingredient is a vitamin or analogue thereof; an occlusive agent; an aqueous solvent; an organic cosolvent; wherein the pharmaceutically active ingredient is insoluble in both water and the occlusive agent; and the occlusive agent being present in an amount sufficient to form an occlusive layer on the skin, in use. In a second embodiment, an oil-in water emulsion having a vitamin, an occlusive agent; an aqueous solvent; and an organic cosolvent, wherein the occlusive agent is present in an amount sufficient to form an occlusive layer on the skin.
Owner:STIEFEL WEST COAST
Vaccine formulations
ActiveUS20050079185A1Improve stabilityStable and safe and easily administrableAntibacterial agentsSsRNA viruses negative-senseEukaryotic plasmidsNon ionic
The present invention provides for a novel oil-in-water (O / W) emulsion, with increased stability in the presence of bacterial or viral suspensions, especially those concentrated and non-purified or weakly purified. The emulsion of the present invention can act as vehicle for the delivery of a pharmaceutical composition comprising at least one immunogen and, in particular, an immunogen selected from the group comprising an inactivated pathogen, an attenuated pathogen, a subunit, a recombinant expression vector, and a plasmid or combinations thereof. In one embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen, said immunogen selected from the group comprising an inactivated Mycoplasma hyopneumoniae bacterium, an inactivated porcine circovirus type 2 (PCV-2) virus or combinations thereof; (2) a mineral oil; (3) a non-ionic lipophilic surfactant; and (4) a non-ionic hydrophilic surfactant having a low HLB value which comprises ethoxylated fatty acid diesters of sorbitan (generally having HLB value between 11 and 13). In another preferred embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen; (2) a non-ionic hydrophilic surfactant having a high hydrophilic-lipophilic balance (HLB) value greater than 13 and less than 40, in particular HLB≧13.5, and preferably HLB≧14; (3) a mineral oil; (4) a non-ionic lipophilic surfactant; and (5) a non-ionic hydrophilic surfactant having a low HLB value (HLB value of about 9 to about 13).
Owner:MERIAL INC
Cosmetic compositions having improved wear and beauty benefits
InactiveUS6503495B1Maintain integrityInhibit growthCosmetic preparationsImpression capsWater insolubleWater soluble
Owner:NOXELL CORP
Topical foam composition
The present invention relates to a novel oil in water emulsion aerosol foam composition containing an active agent for the treatment of various chronic and acute skin conditions, particularly acne and psoriasis; and processes for preparing the emulsion aerosol foam compositions. In particular, the present invention relates to oil in water emulsion aerosol foam compositions containing a retinoid in the oil phase.
Owner:MAYNE PHARMA LLC
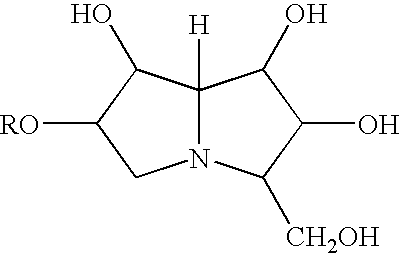
Continuous multi-microencapsulation process for improving the stability and storage life of biologically active ingredients
The invention relates to microcapsules, and a continuous micro-encapsulation water-in-oil-in-water microencapsulation process through in situ and interfacial polymerization of the emulsion. The formulation comprises a continuous water phase having a dispersion of microcapsules which contain oil drops and wherein the inside of each oil phase drop -containing optionally oil-soluble materials- there is a dispersion of water, or aqueous extract or water dispersible material or water soluble material. The oil drops are encapsulated with a polymerisable material of natural origin. Such microcapsules are appropriated for spray-dry processes, to be used as dry powder, lyophilised, self-emulsifiable powder, gel, cream and any liquid form. The active compounds included in te microcapsules are beneficial to the health and other biological purposes. Such formulations are appropriate to be incorporated in any class of food, specially for the production of nutraceuticals, as well as cosmetic products (such as rejuvenescence creams, anti-wrinkle creams, gels, bath and shower consumable products and sprays). The preparations are adequate to stabilise compounds added to the food, media for cultivating microbes and nutraceuticals, specially those which are easily degradable or oxidizable.
Owner:CHEM FAB BUDENHEIM AG
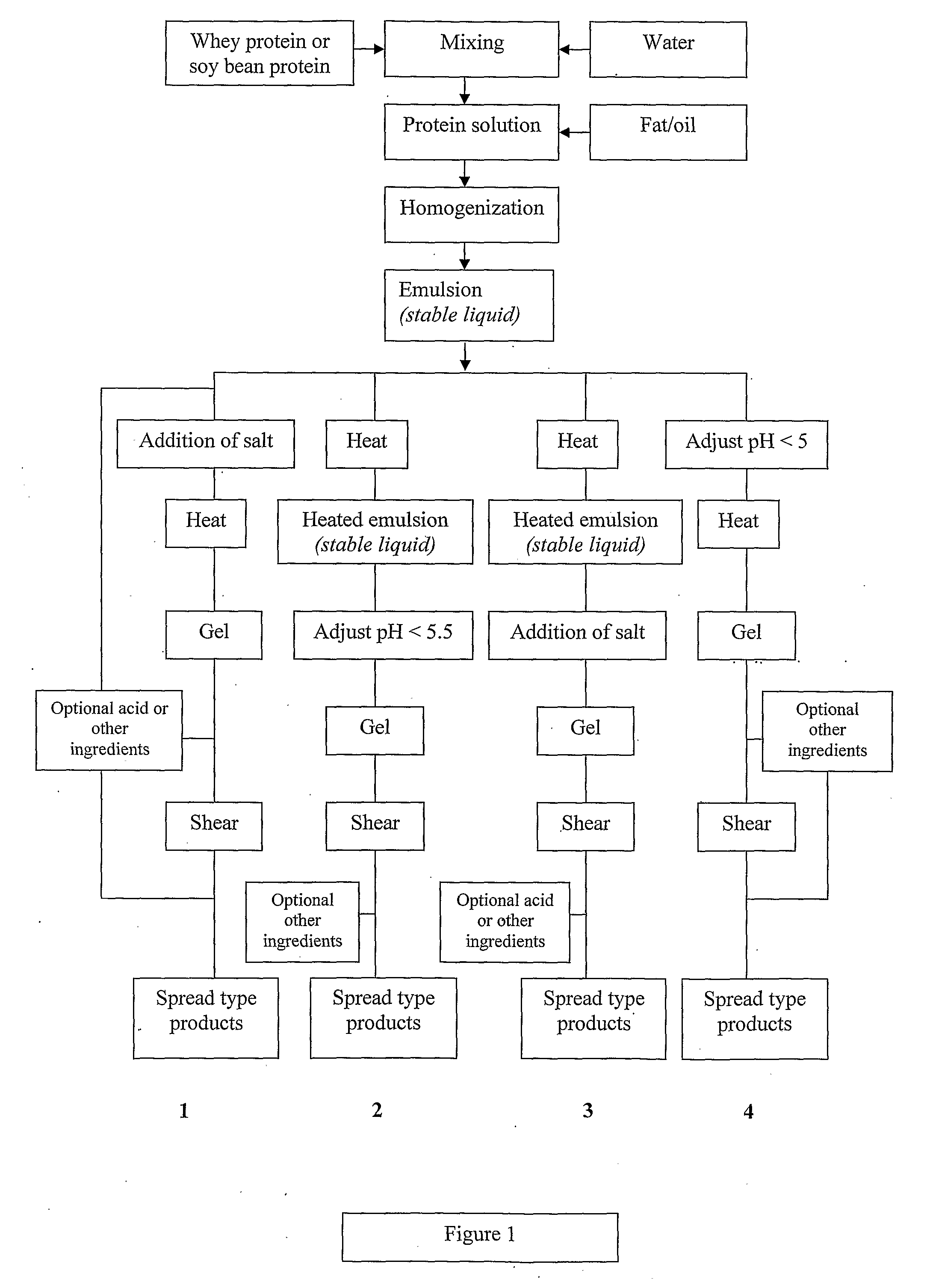
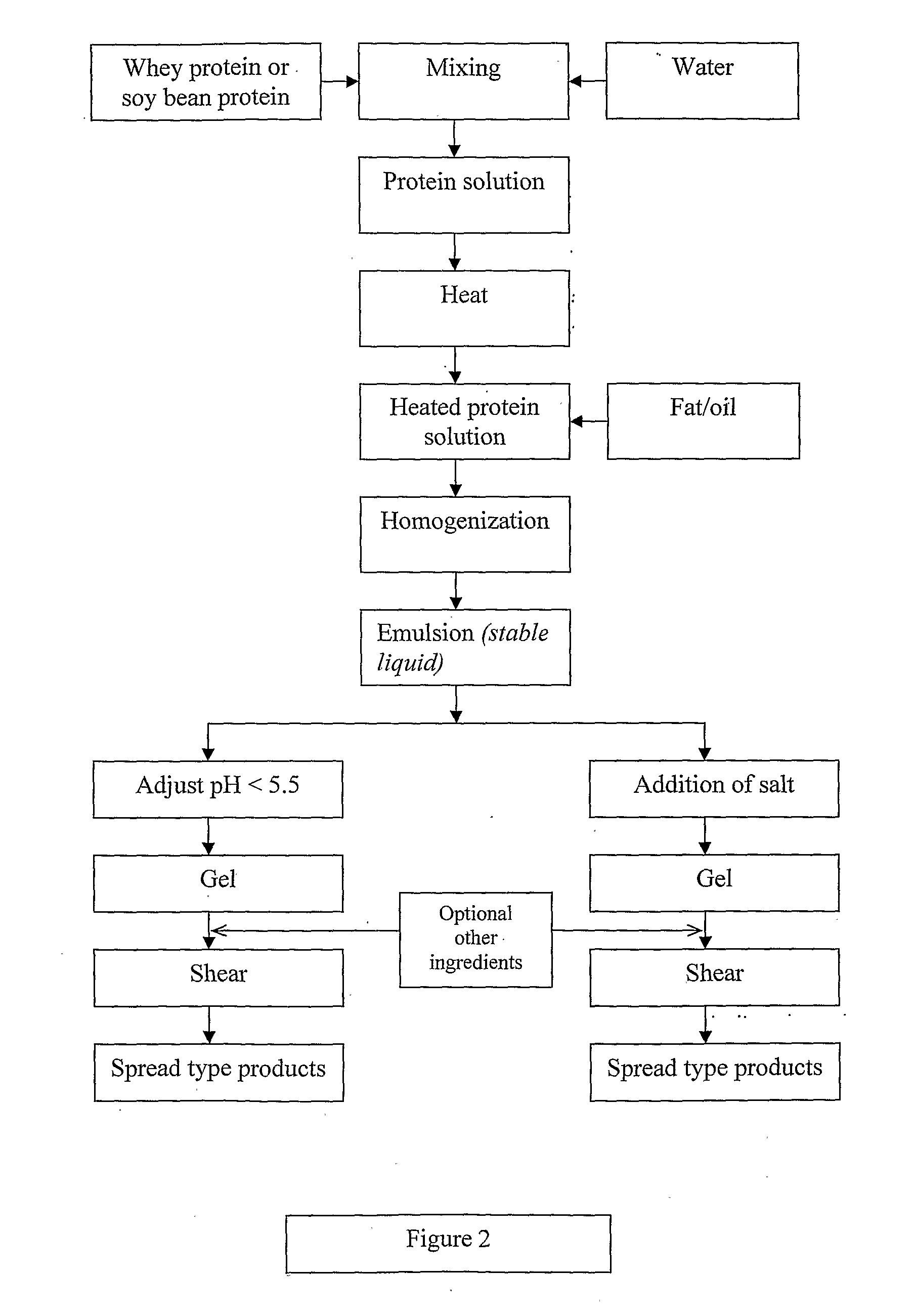
Dairy product and process
InactiveUS20100143567A1Lower pHLow viscosityMilk preparationEdible oils/fats ingredientsWhey proteinHeat treated
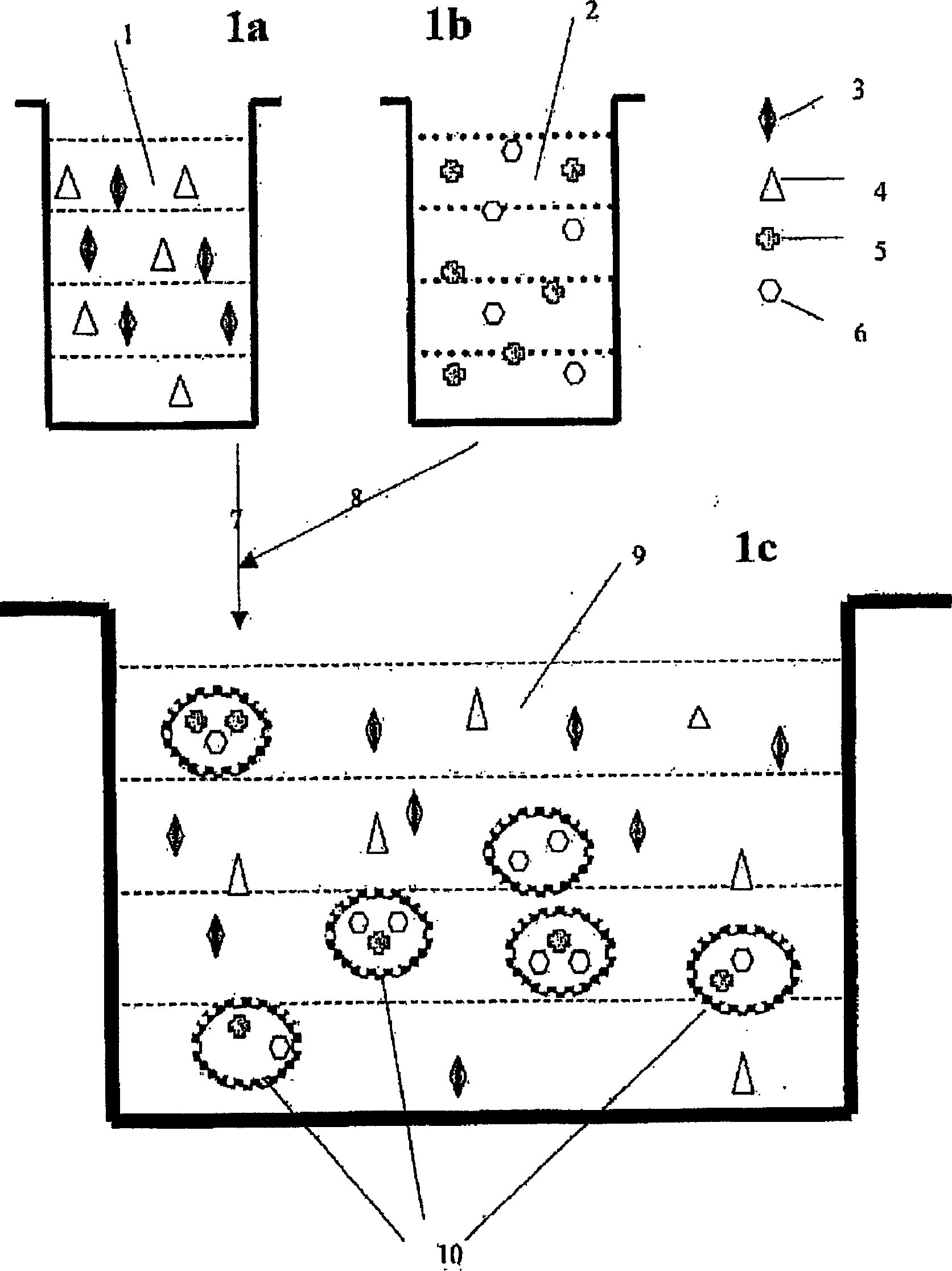
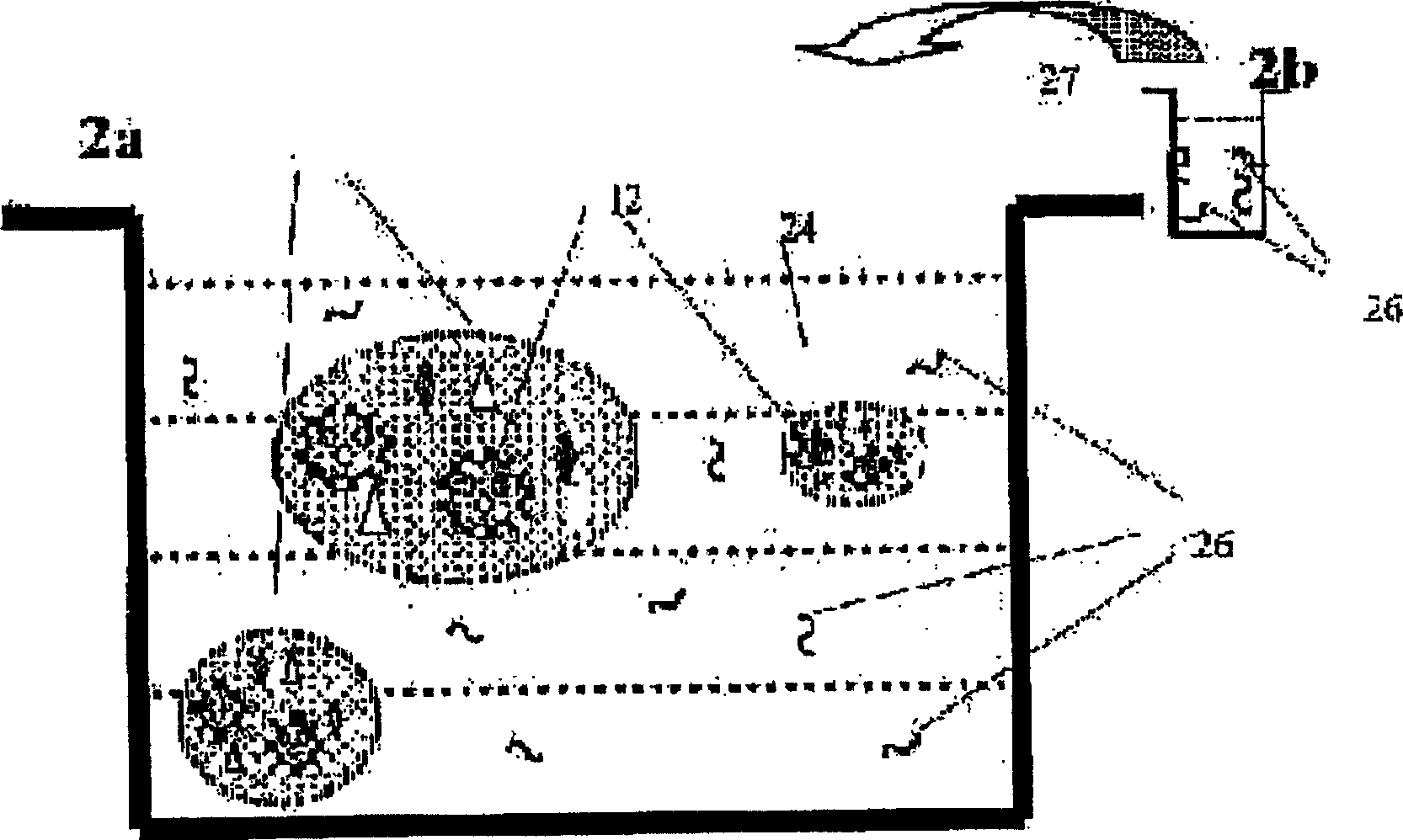
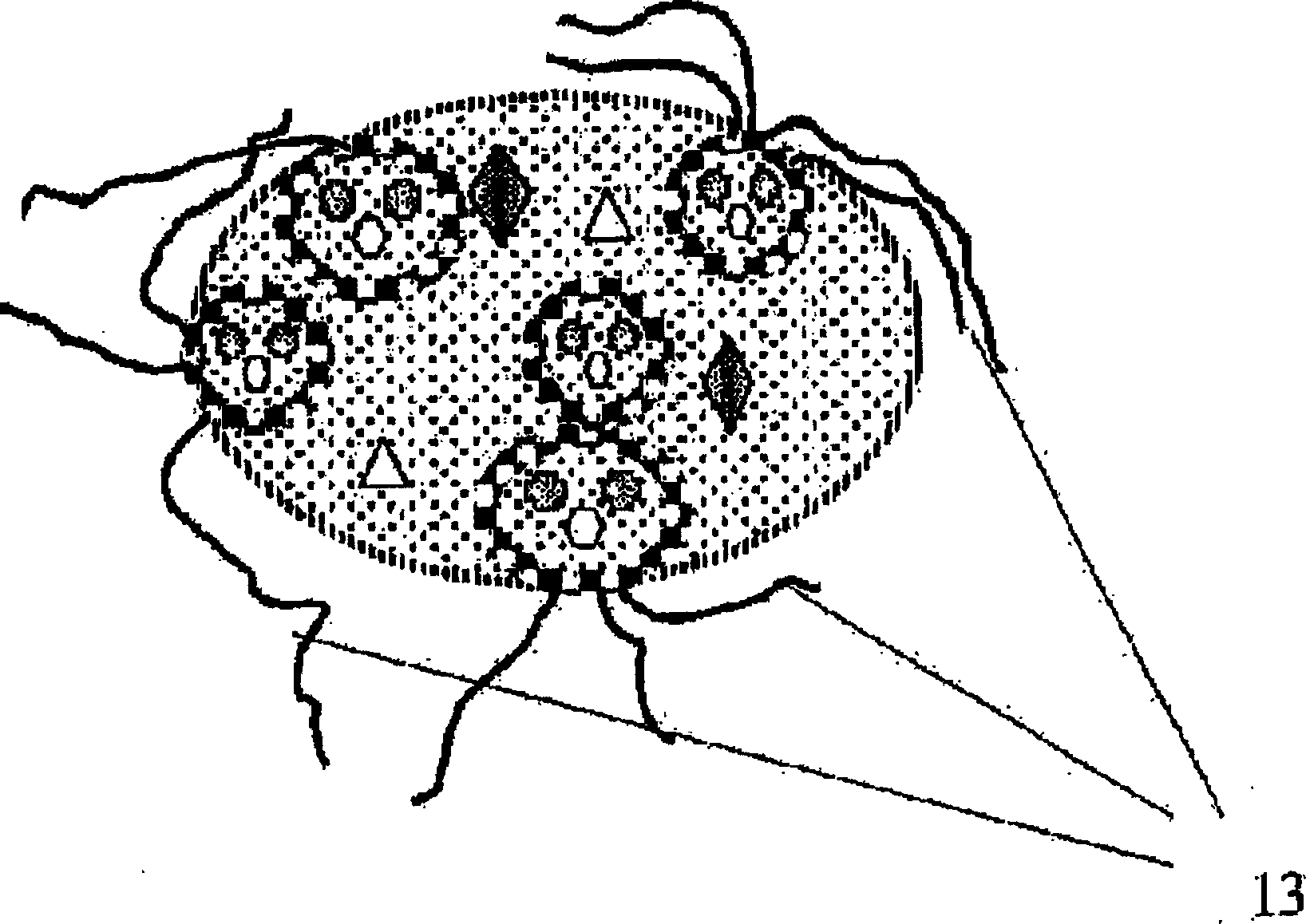
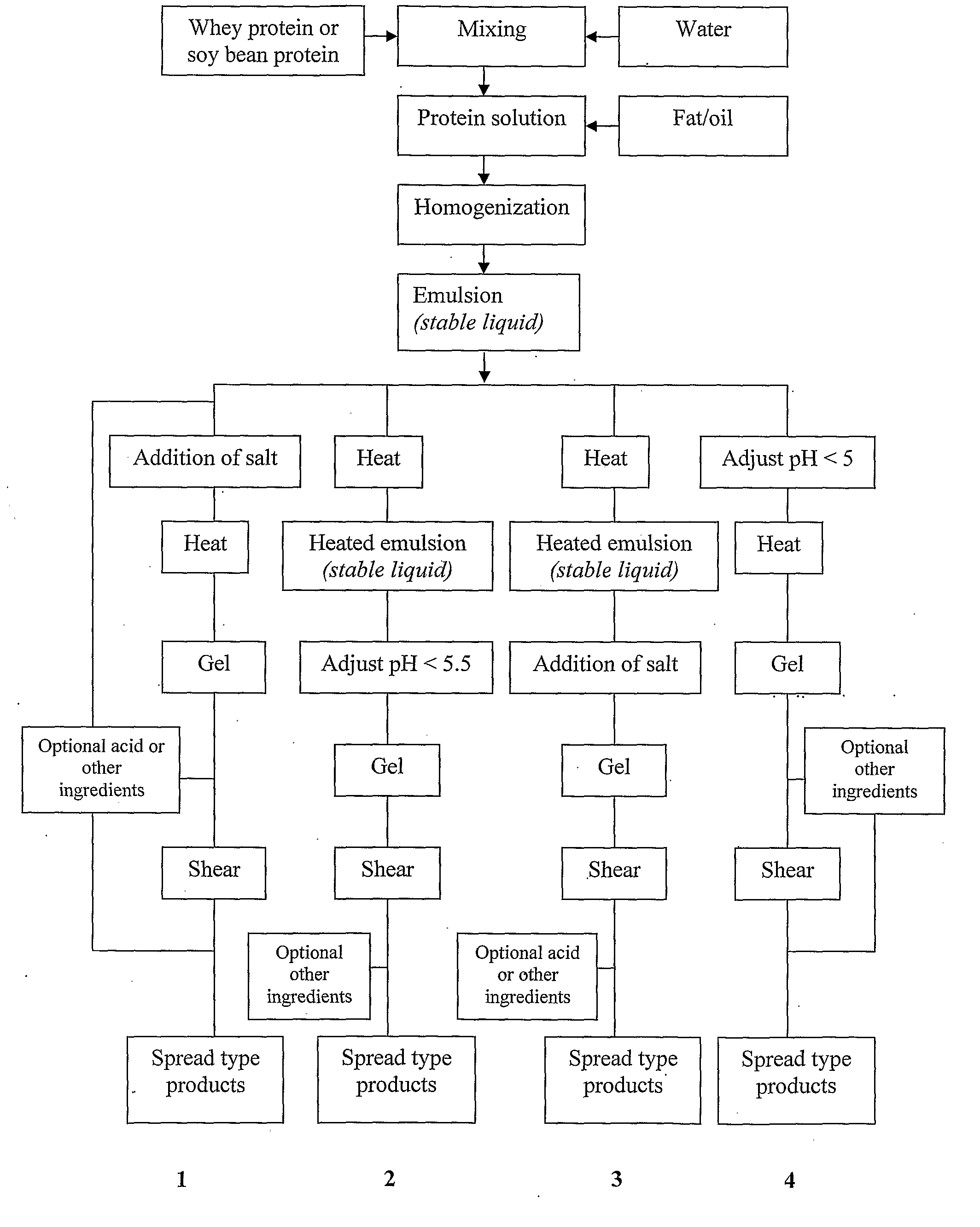
A viscoelastic fluid is prepared by shearing a gelled emulsion. The emulsion is an oil-in-water emulsion comprising 2% 12% (w / w) of a heat-treated protein that can form a heat-set gel and 5% 40% fat. The proteins used include whey protein and soy protein. The viscoelastic fluid may be used as a spread. It may also be used for the preparation of a plurality of different ultimate products formed from the original gel.
Owner:FONTERRA COOP GRP LTD
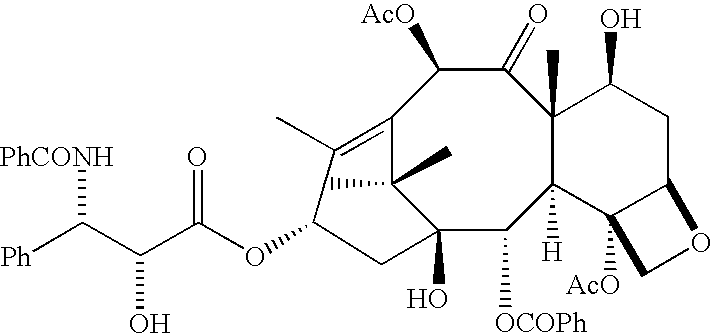
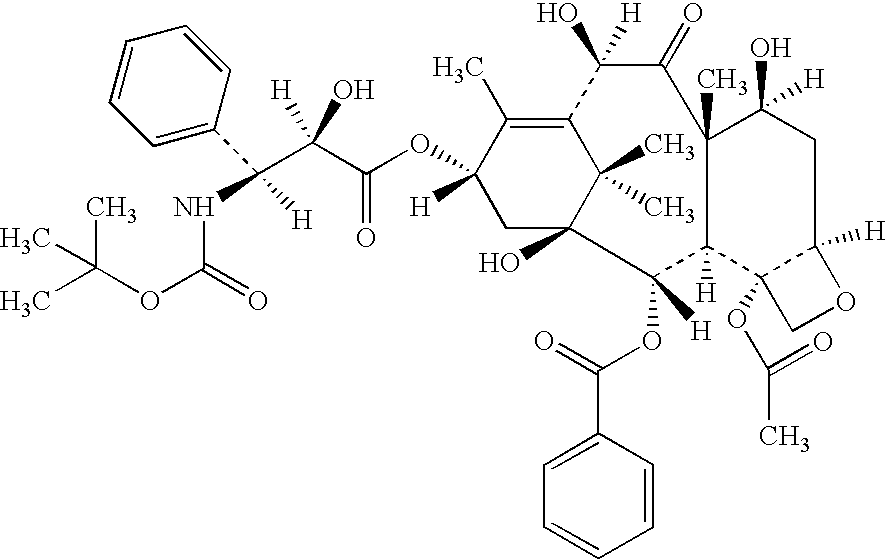
Low oil emulsion compositions for delivering taxoids and other insoluble drugs
ActiveUS20060067952A1Reduce oil contentNot hyperallergenicOrganic active ingredientsBiocideOil emulsionWater insoluble
The present invention provides injectable oil-in-water emulsions of taxoid drugs or other water insoluble drugs. The present invention also provides methods for preparing and using such oil-in-water emulsions.
Owner:CHEN ANDREW XIAN
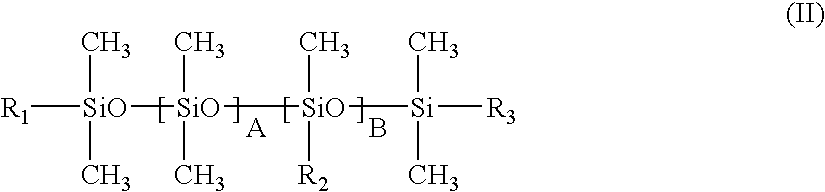
Composition in the form of an oil-in water emulsion containing a silicone copolymer and showing a liquid crystalline phase and uses thereof
InactiveUS7087650B2Good cosmetic effectGood flexibilityCosmetic preparationsHair cosmeticsLiquid crystallineSURFACTANT BLEND
A composition in the form of an oil-in-water emulsion containing, in a physiologically acceptable medium, an oily phase dispersed in an aqueous phase, the aqueous phase containing particles of a noncrosslinked silicone copolymer and at least one amphiphilic surfactant capable of forming liquid crystals.
Owner:LOREAL SA
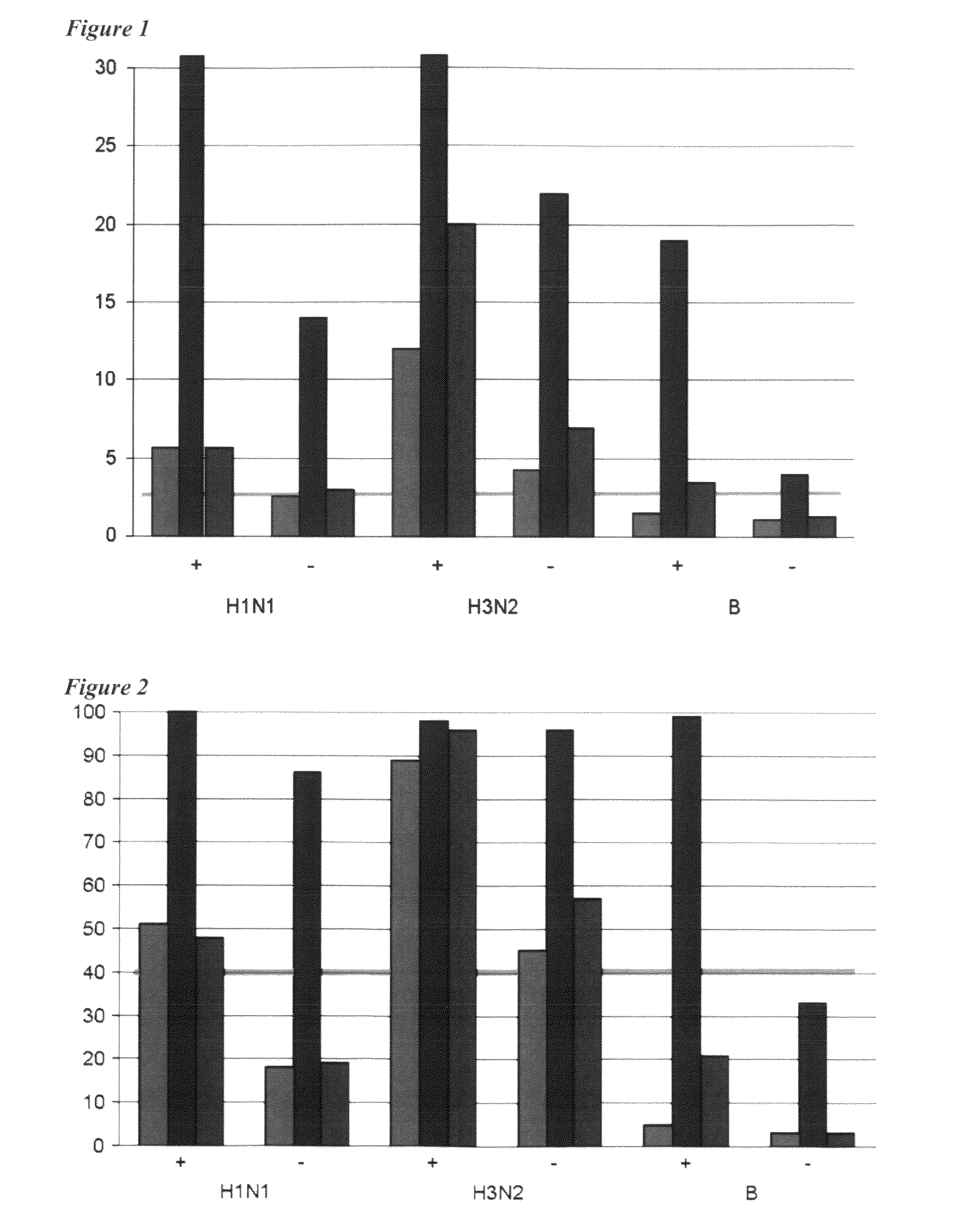
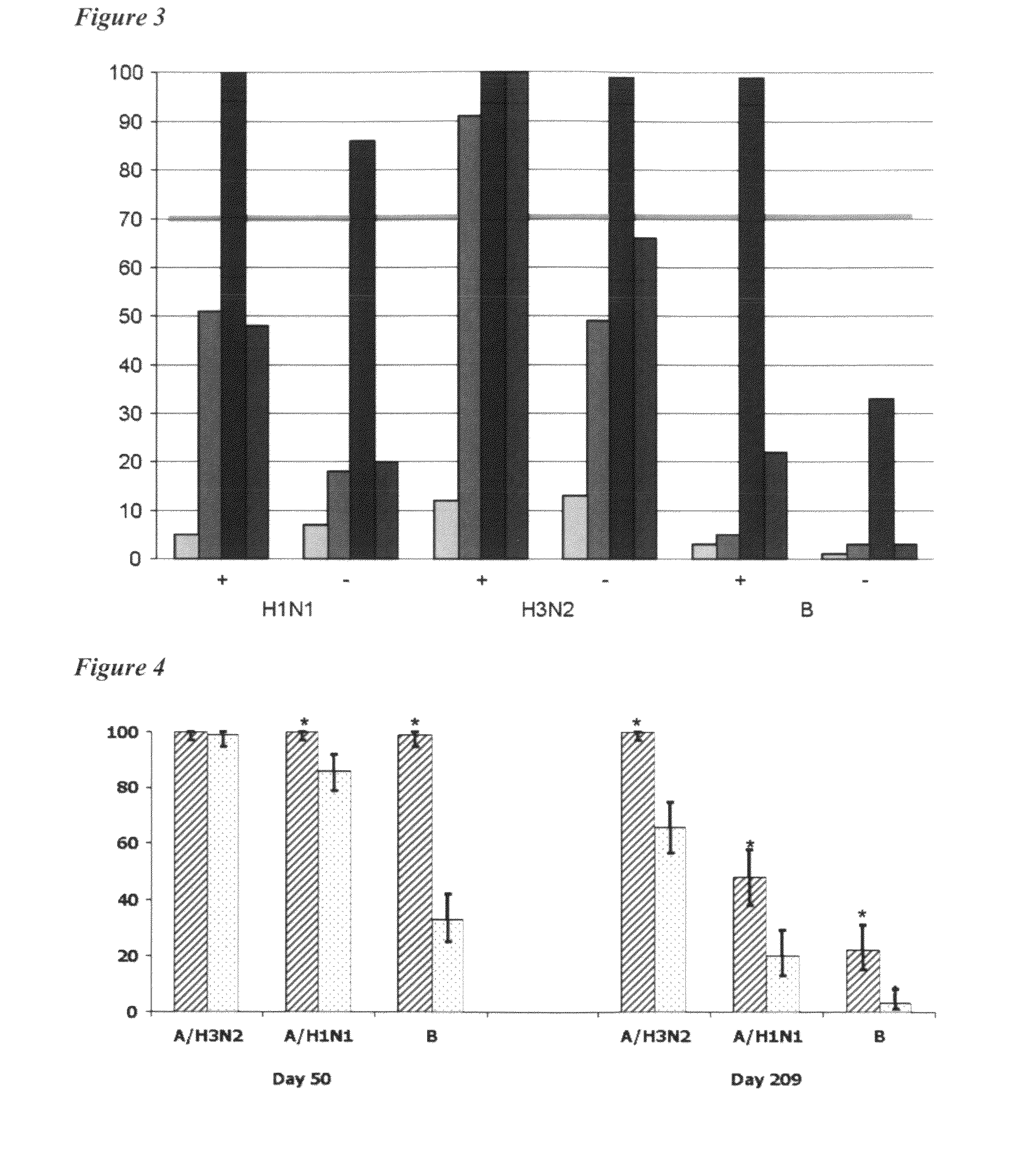
Adjuvanted influenza vaccines for pediatric use
ActiveUS8506966B2Enhance immune responseHigh seroprotection rateSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSeroconversion
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
Nanoemulsion containing a hydroxylated urea compound
InactiveUS20060193813A1High transparencyIncrease viscosity of fluidCosmetic preparationsHair removalLipid formationAverage size
The invention relates to an oil-in-water nanoemulsion having an oily phase dispersed in an aqueous phase, the oil globules of which have a number-average size of less than 100 nm, containing: (i) at least one amphiphilic lipid comprising at least one nonionic amphiphilic lipid, and optionally at least one ionic amphiphilic lipid, the oily phase and the amphiphilic lipid being present at a content such that the oily phase / amphiphilic lipid weight ratio ranges from 3 to 10, and (ii) at least one hydroxylated urea derivative. Application in cosmetics, dermatology, and ophthalmology.
Owner:LOREAL SA
Cpg formulations and related methods
The invention involves methods and compositions of an immunostimulatory nucleic acid in combination with other therapeutic formulations such as oil-in-water emulsions. The combination of therapeutics are administered in various dosages or at various time schedules for the treatment of disorders such as disease and cancer.
Owner:MERIAL LTD
Oil-in-water personal care composition
InactiveUS20080181956A1Good skin feelImprove skinPowder deliveryCosmetic preparationsPersonal careMedicine
A personal care composition in the form of an oil-in-water emulsion is disclosed that provides improves skin feel. The personal care composition comprises from a silicone elastomer; a silicone fluid; a particulate material having a refractive index of less than about 1.8; a polymeric thickener; and water. The personal care composition may be substantially devoid of opacifying particulate material. A consumer product comprising the personal care composition is also disclosed. The consumer product allows for the application of the personal care composition to the skin or other keratinous tissue.
Owner:THE PROCTER & GAMBLE COMPANY
Topical Therapeutic Delivery System
The present invention relates to an oil-in-water emulsion topical delivery system comprising an oil phase; an aqueous phase; phenoxyethanol; an effective exfoliatingamount of a hydrophobic hydroxycarboxylic acid; a non-ionic emulsifier having an HLB of from about 7 to about 10; and at least one skin-supporting ordermatopharmaceutically active agent.
Owner:MURAD HOWARD
Novel vaccine formulations
ActiveUS20060233831A1Improve stabilityStable and safe and easily administrableAntibacterial agentsBiocideAdjuvantNon ionic
The present invention relates to oil-in-water emulsions, their use as adjuvants, and pharmaceutical, immunologic, or vaccine compositions that may comprise the same. In one embodiment, the oil-in-water (O / W) emulsion may comprise an aqueous solution containing an immunogen, a mineral oil, a non-ionic lipophilic ethoxylated fatty alcohol and a non-ionic hydrophilic surfactant. In another embodiment, the oil-in-water (O / W) emulsion may comprise an aqueous solution containing an immunogen, a non-ionic lipophilic surfactant, a mineral oil and a non-ionic hydrophilic ethoxylated fatty alcohol. The present invention also encompasses a method of making a vaccine composition using the adjuvant of the instant invention, the vaccine composition so obtained and methods of use.
Owner:MERIAL INC
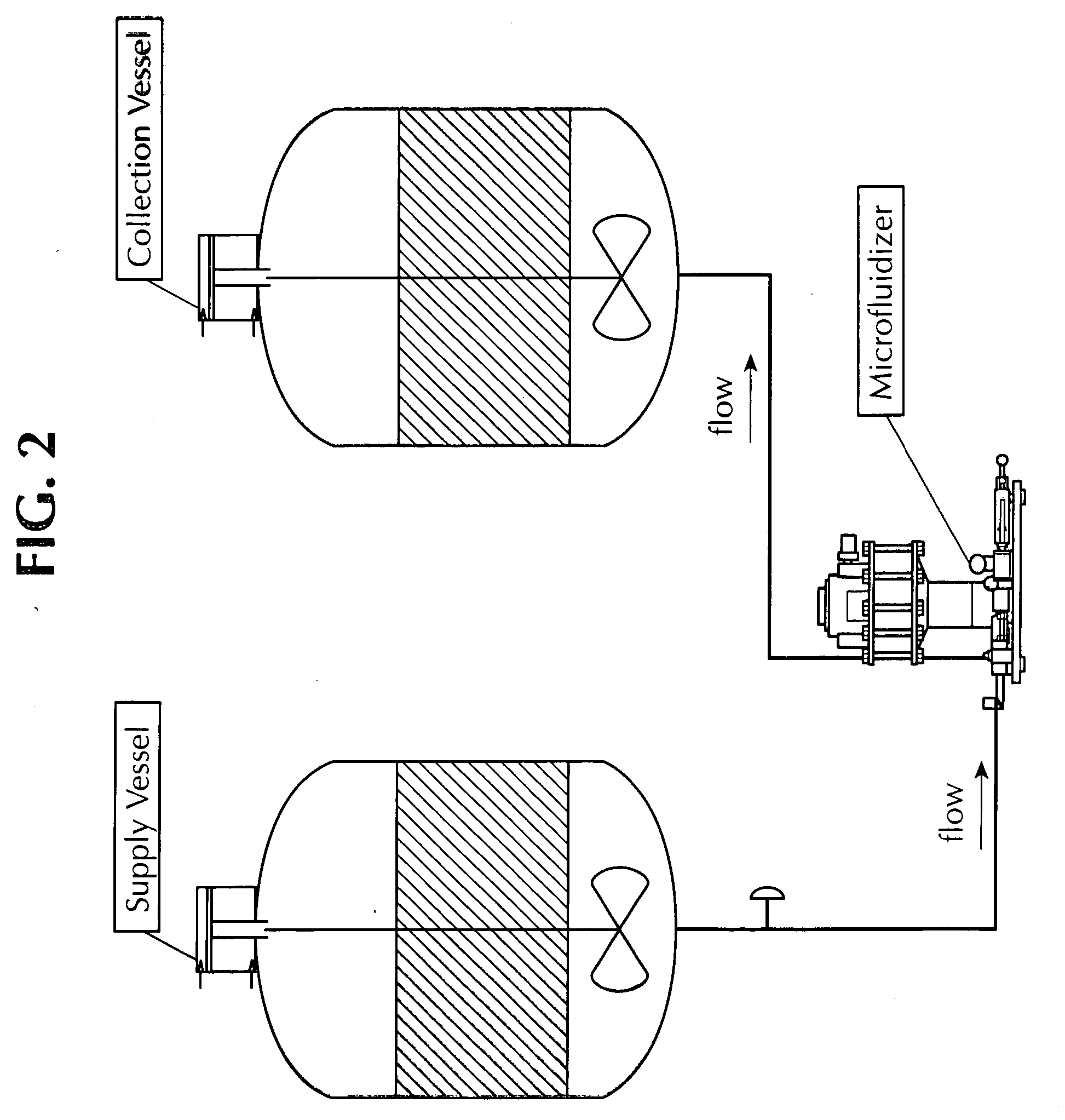
Microfluidized oil-in-water emulsions and vaccine compositions
This invention provides submicron oil-in-water emulsions useful as a vaccine adjuvant for enhancing the immunogenicity of antigens. The present invention also provides vaccine compositions containing an antigen combined with such emulsions intrinsically or extrinsically. Methods of preparing the emulsions and vaccines are also provided by the present invention.
Owner:ZOETIS SERVICE LLC
Microemulsions for use in food and beverage products
ActiveUS20070087104A1Acceptable tasteImprove bioavailabilityEdible oils/fats with aqeous phaseFood gradeBeta-Carotene
Oil-in-water microemulsions which can be used to incorporate lipophilic water-insoluble materials, such as beta-carotene, into food and beverage compositions are disclosed. The microemulsions utilize a ternary food grade emulsifier system which incorporates a low HLB emulsifier, a medium HLB emulsifier, and a high HLB emulsifier. The microemulsions of the present invention allow for the incorporating of water-insoluble materials in an effective and easy-to-process manner while providing formulational flexibility and without significantly affecting the taste of the underlying food or beverage product. Food and beverage products including the microemulsions are also disclosed. Finally, the method of preparing the microemulsions is described. The invention also encompasses water-in-oil microemulsions for use in incorporating water-soluble materials into food and beverage products. Finally, the invention encompasses concentrate compositions used for making those microemulsions.
Owner:WILD FLAVORS INC
Intra-serum and intra-gel for modeling human skin tissue
InactiveUS6475800B1Diagnostics using spectroscopyScattering properties measurementsConfocalCrosslinking reagent
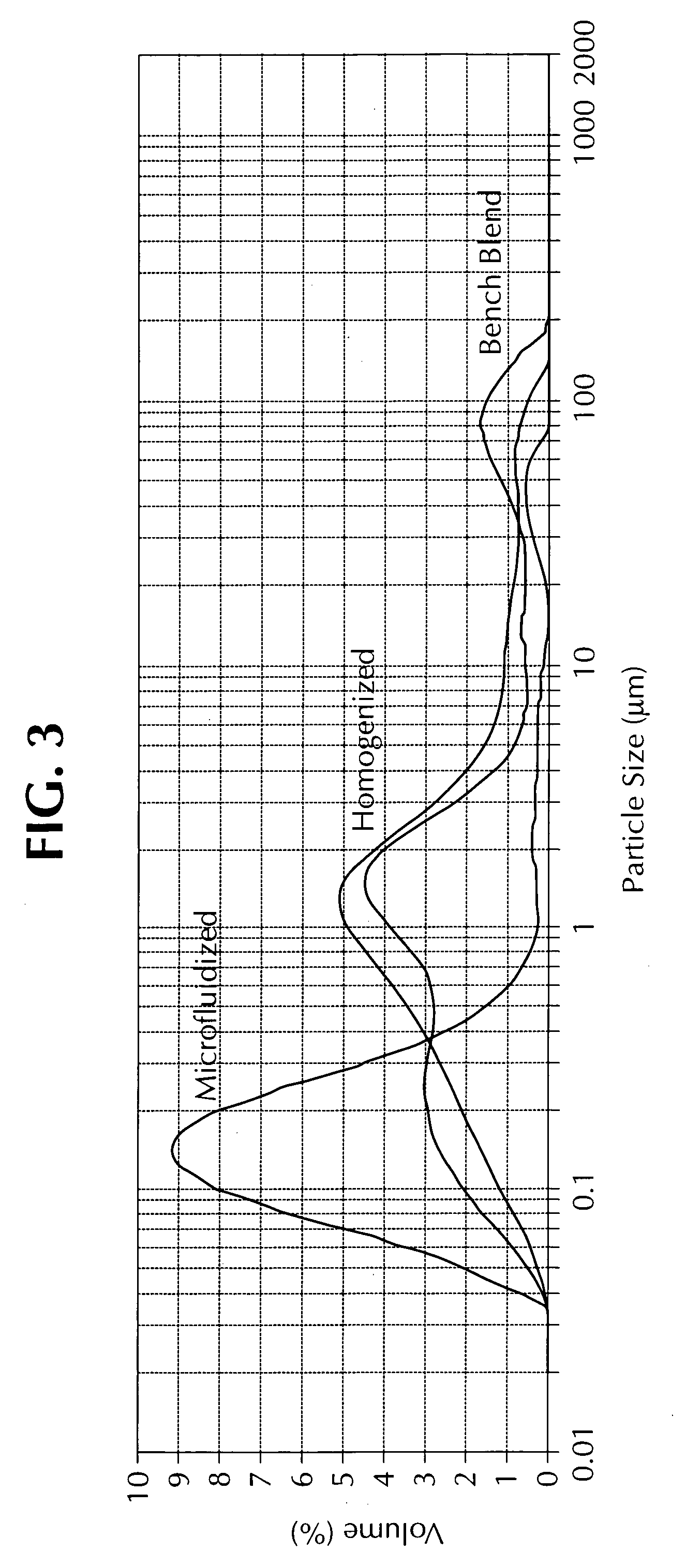
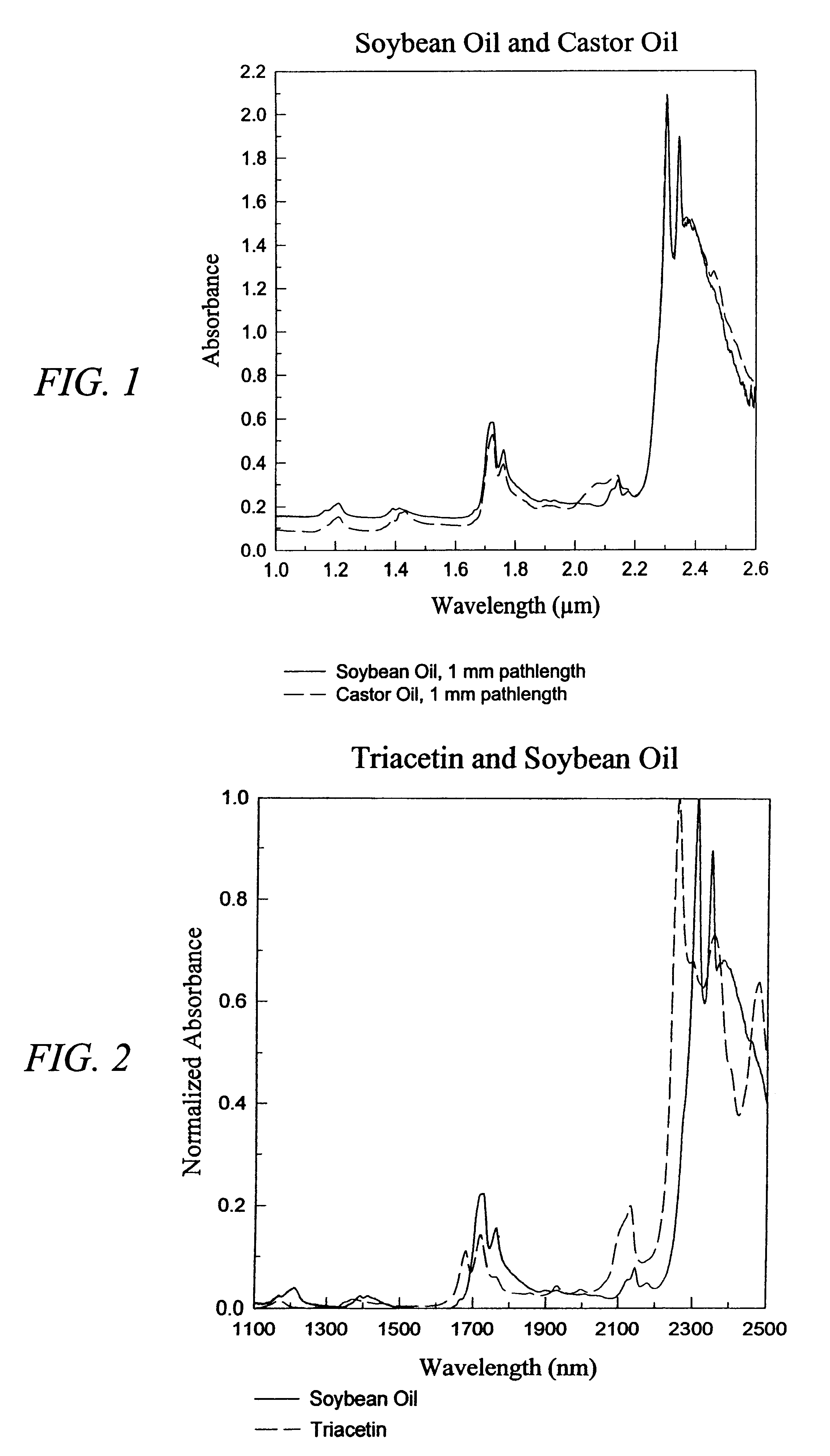
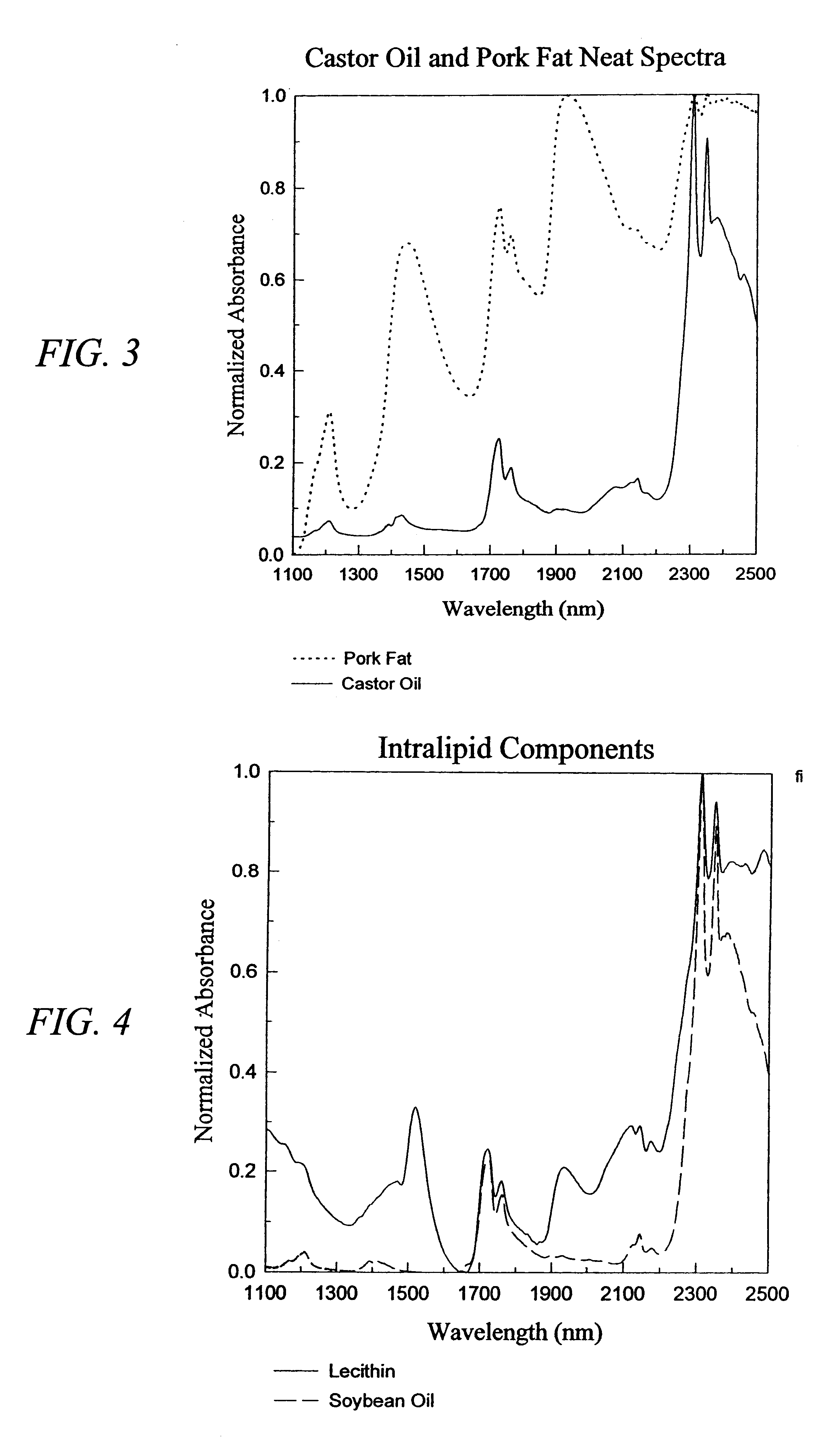
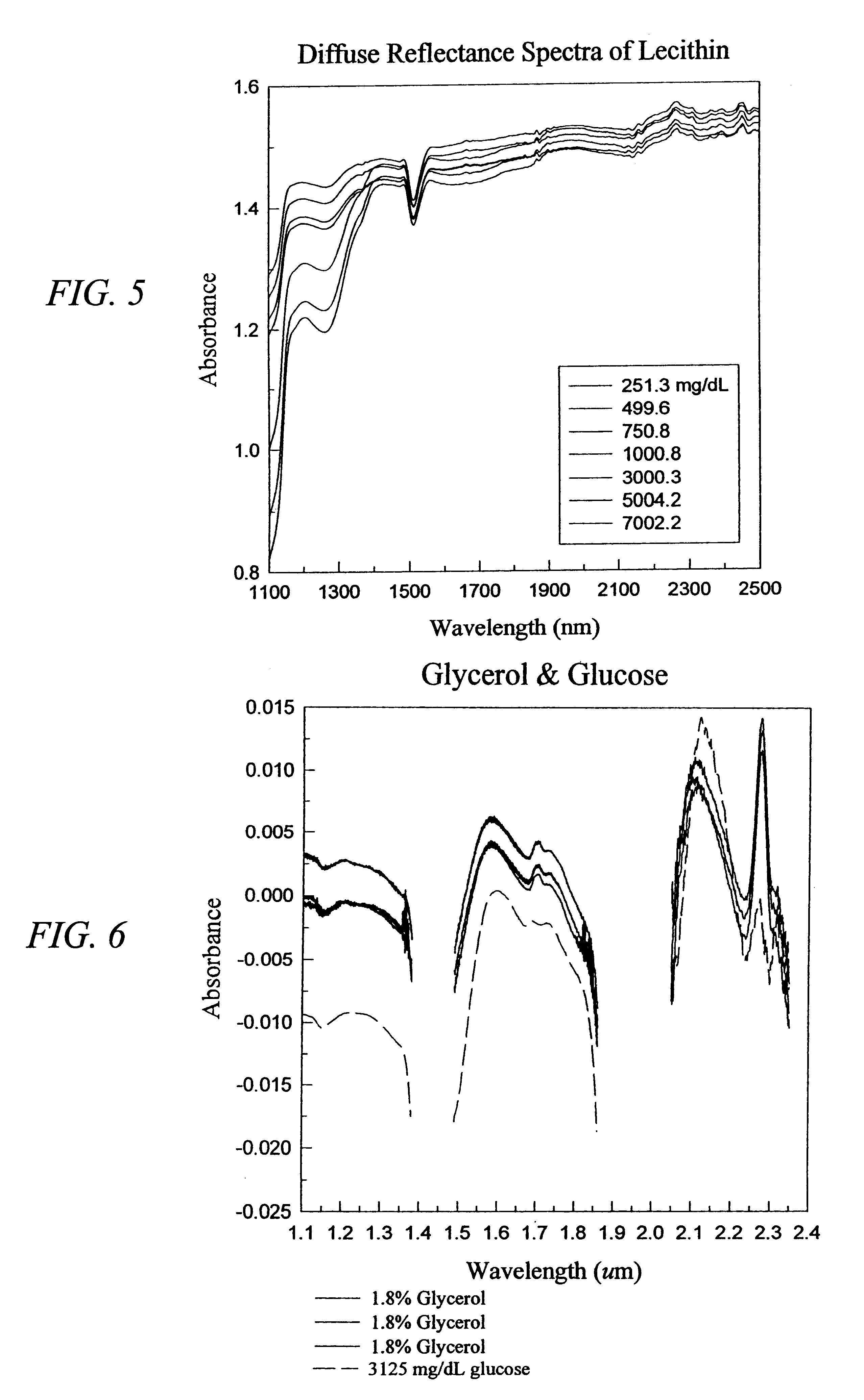
The invention provides a class of samples that model the human body. This family of samples is based upon emulsions of oil in water with lecithin acting as the emulsifier. These solutions that have varying particle sizes may be spiked with basis set components (albumin, urea and glucose) to simulate skin tissues further. The family of samples is such that other organic compounds such as collagen, elastin, globulin and bilirubin may be added, as can salts such as Na+, K+ and Cl-. Layers of varying thickness with known index of refraction and particle size distributions may be generated using simple crosslinking reagents, such as collagen (gelatin). The resulting samples are flexible in each analyte's concentration and match the skin layers of the body in terms of the samples reduced scattering and absorption coefficients, mums and muma. This family of samples is provided for use in the medical field where lasers and spectroscopy based analyzers are used in treatment of the body. In particular, knowledge may be gained on net analyte signal, photon depth of penetration, photon radial diffusion, photon interaction between tissue layers, photon density (all as a function of frequency) and on instrument parameter specifications such as resolution and required dynamic range (A / D bits required). In particular, applications to delineate such parameters have been developed for the application of noninvasive glucose determination in the near-IR region from 700 to 2500 nm with an emphasis on the region 1000 to 2500 nm (10,000 to 4,000 cm-1).
Owner:GLT ACQUISITION
Topically applicable oil-in-water emulsions and dermatological applications thereof
InactiveUS20080207570A1Satisfactory emollient capacityNon-greasy feelOrganic active ingredientsBiocideDiseaseActive agent
Novel topically applicable oil-in-water emulsions, with a high proportion of oily phase in the inner phase, combine the occlusive and emollient properties of an ointment without having the drawbacks of a greasy feel, while at the same time promoting the therapeutic properties of a biologically active agent contained therein; such emulsions are especially useful for the treatment of dermatological diseases, conditions or afflictions, notably psoriasis.
Owner:GALDERMA SA
Cationic oil-in-water emulsions
ActiveUS20140220083A1Emulsion stabilizationSsRNA viruses negative-senseOrganic active ingredientsHigh concentrationMolecular interactions
This invention generally relates to cationic oil-in-water emulsions that contain high concentrations of cationic lipids and have a defined oil:lipid ratio. The cationic lipid can interact with the negatively charged molecule thereby anchoring the molecule to the emulsion particles. The cationic emulsions described herein are useful for delivering negatively charged molecules, such as nucleic acid molecules to cells, and for formulating nucleic acid-based vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Topical compositions and methods for treating pain
InactiveUS20030082214A1Treating and preventing painAvoid painBiocideNervous disorderPreventing painNR1 NMDA receptor
Topical compositions and methods for treating pain. The invention provides oil-in-water emulsions comprising an antidepressant; an NMDA-receptor antagonists; a lipophilic component; water; and a surfactant. The compositions induce a local-anesthetic effect when topically administered to intact skin thereby treating or preventing pain, for example, neuropathic pain.
Owner:EPICEPT CORP
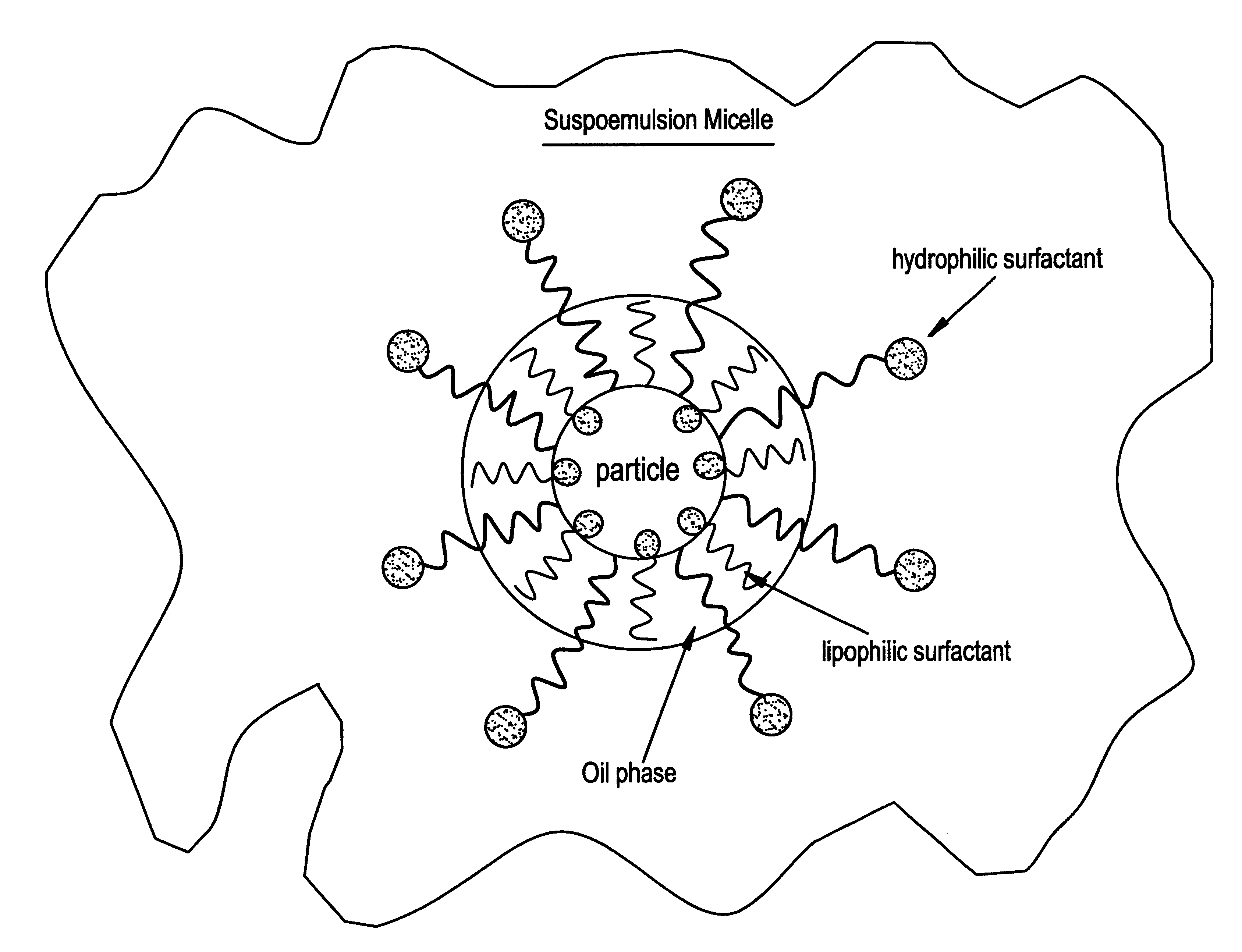
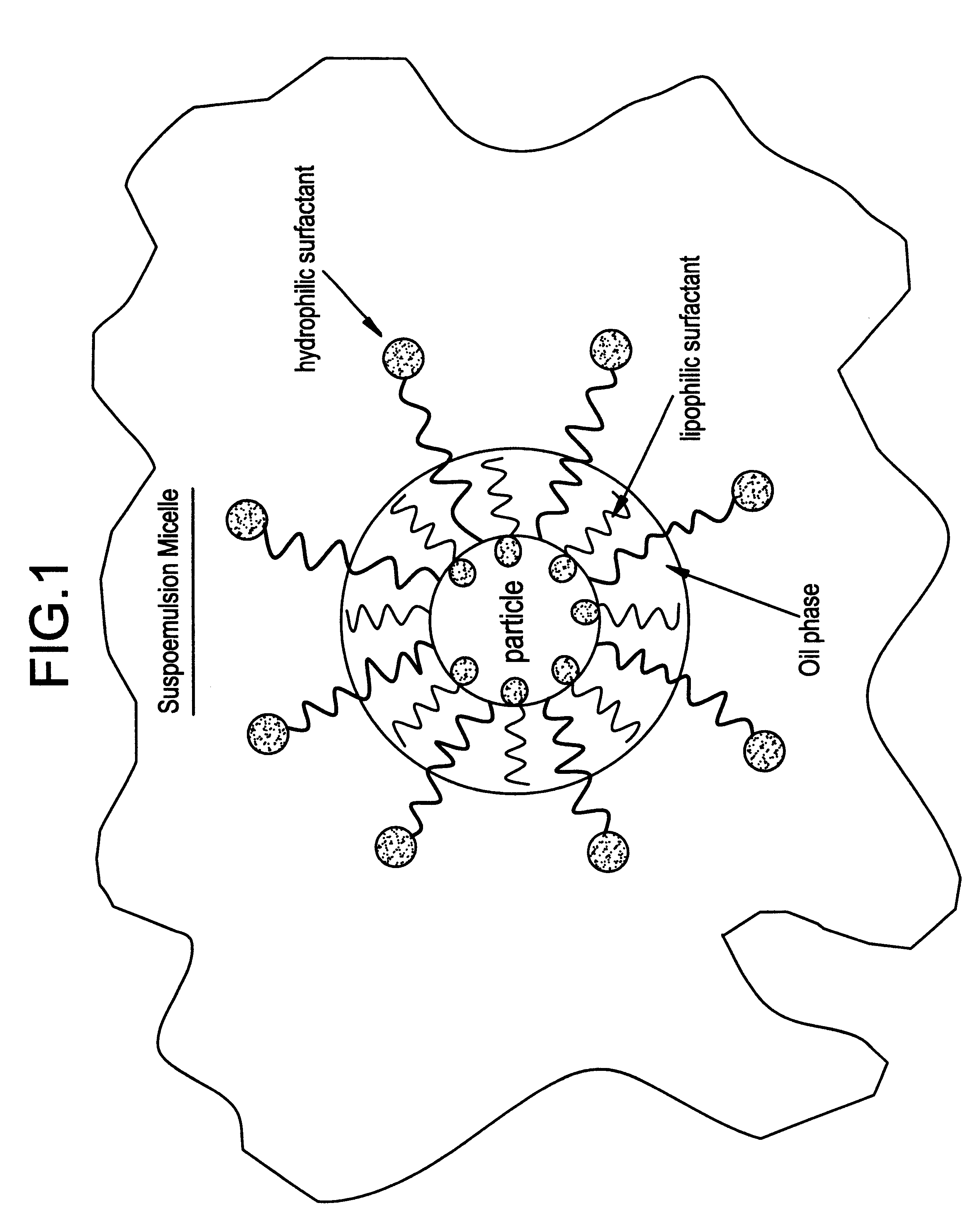
Suspoemulsion system for delivery of actives
InactiveUS6358909B1Heavy loadInorganic/elemental detergent compounding agentsCationic surface-active compoundsOrganic chemistryChemistry
An oil-in-water suspoemulsion system is provided for the delivery of actives for laundering, cleaning or surface treatment, in which the suspoemulsion includes a major portion of water as a continuous phase, at least one Active, and an encapsulate including an oil, and at least first and second nonionic surfactants, the first and second nonionic surfactants having a HLB of at least about 3, the encapsulate substantially completely coating the active and suspending it within the aqueous phase.
Owner:THE CLOROX CO
Composition for wet wipes that enhances the efficacy of cleansing while being gentle to the skin
The present invention describes an oil-in-water emulsion composition for wet-wipes delivering an improved body cleansing performance while providing a gentle and smooth feeling to the user.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com