Patents
Literature
31 results about "Seroconversion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
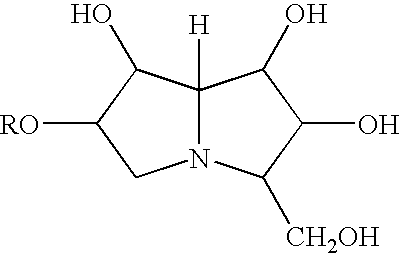
In immunology, seroconversion is the time period during which a specific antibody develops and becomes detectable in the blood. After seroconversion has occurred, the disease can be detected in blood tests for the antibody. During an infection or immunization, antigens enter the blood, and the immune system begins to produce antibodies in response. Before seroconversion, the antigen itself may or may not be detectable, but the antibody is, by definition, absent. During seroconversion, the antibody is present but not yet detectable.
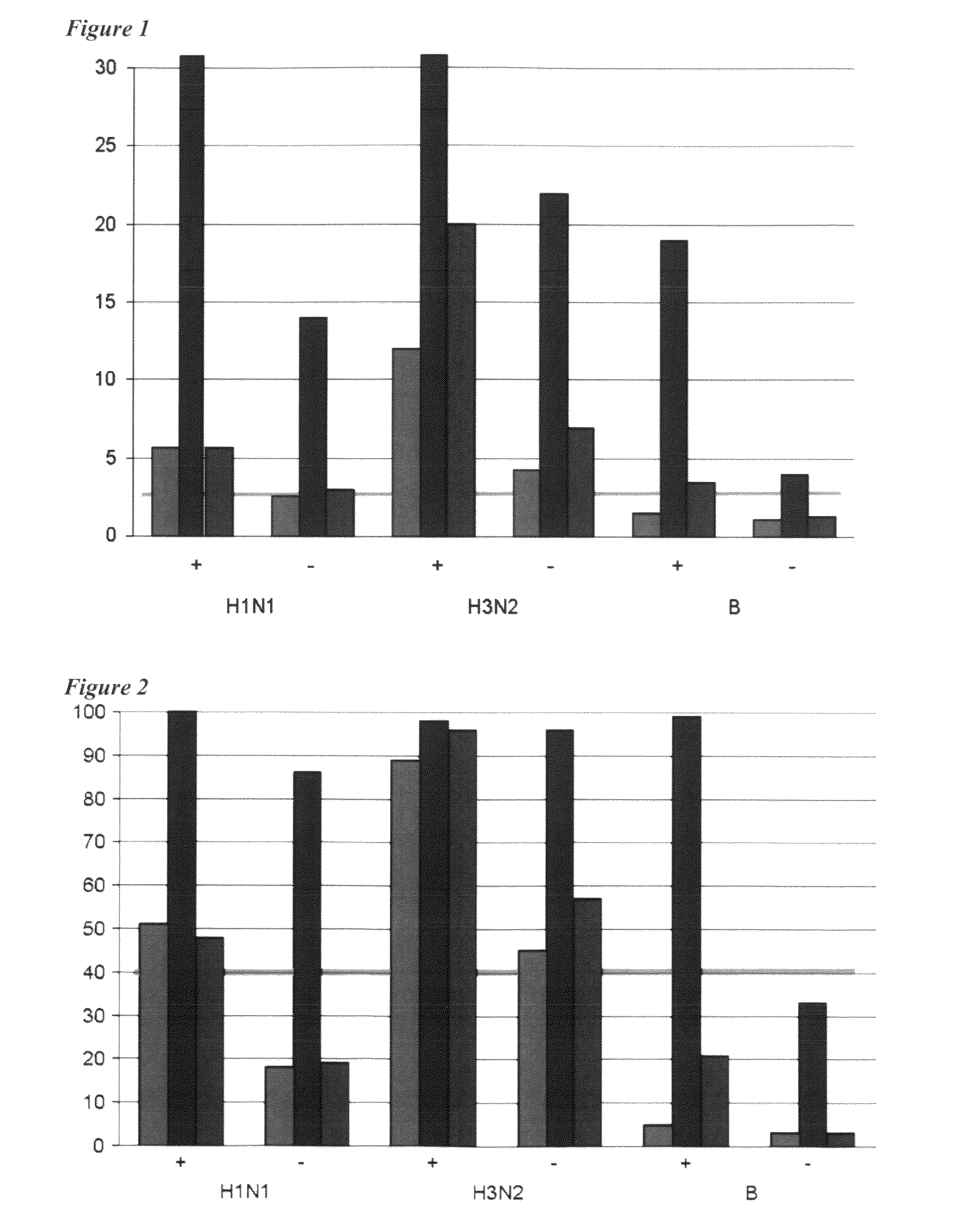
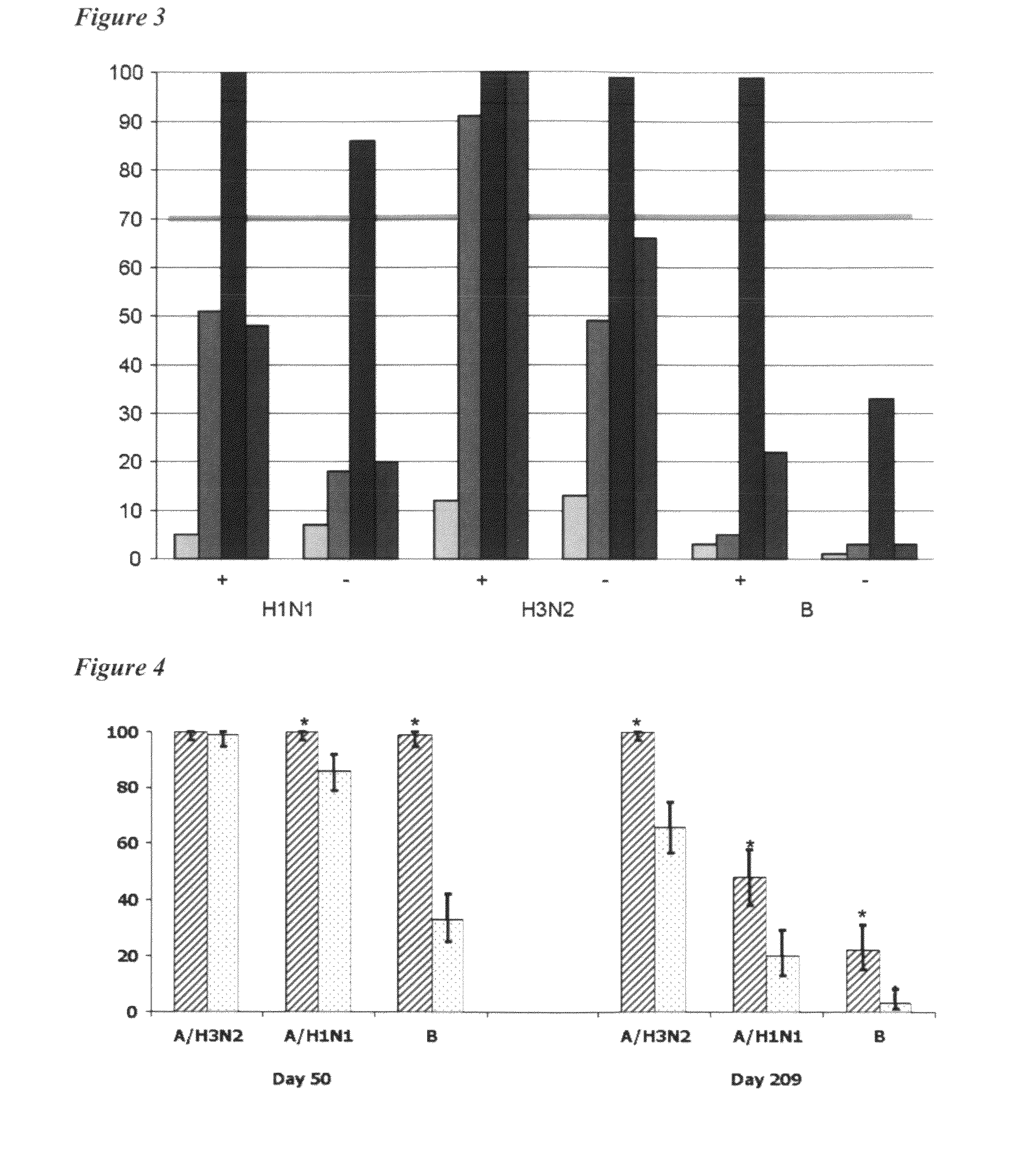
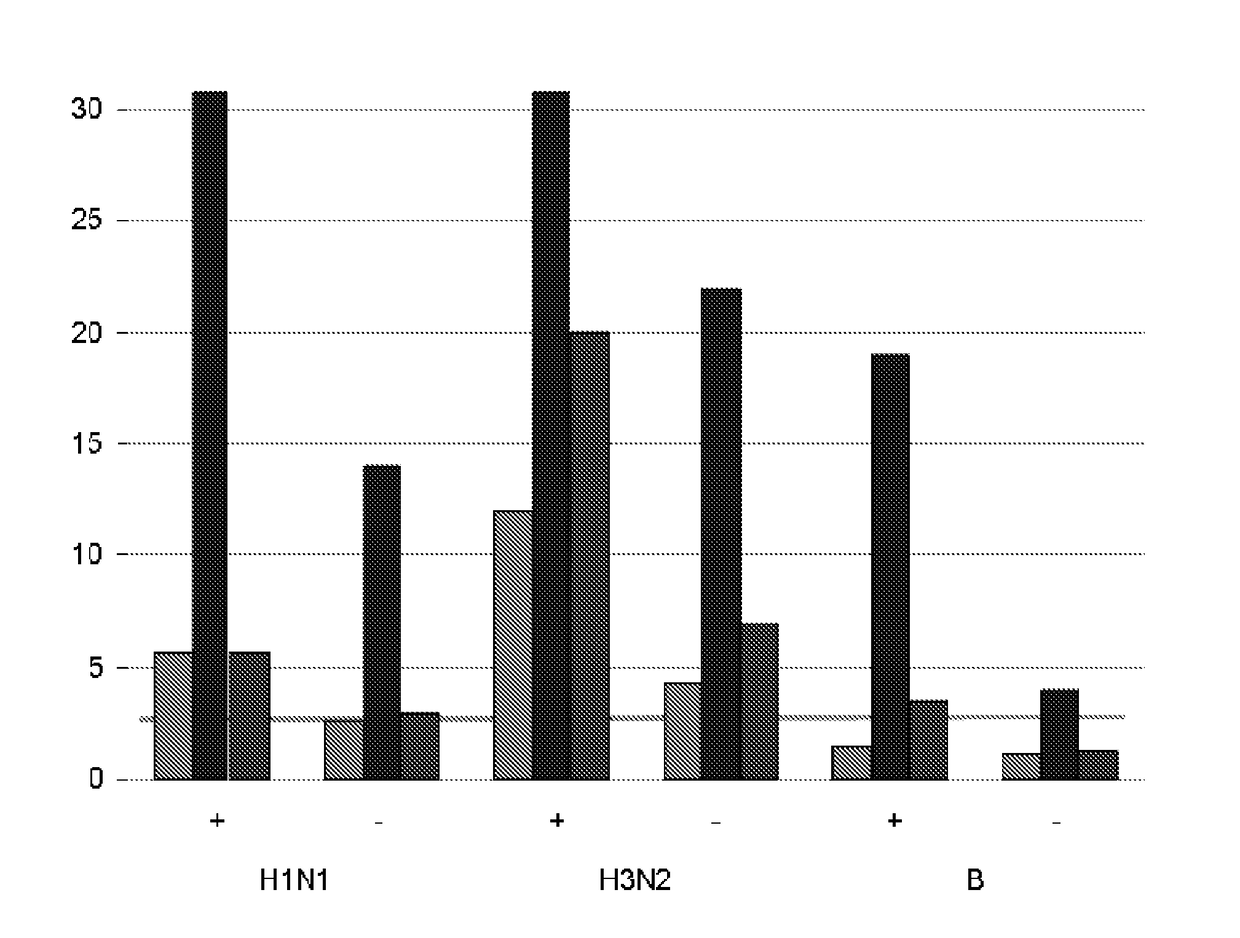
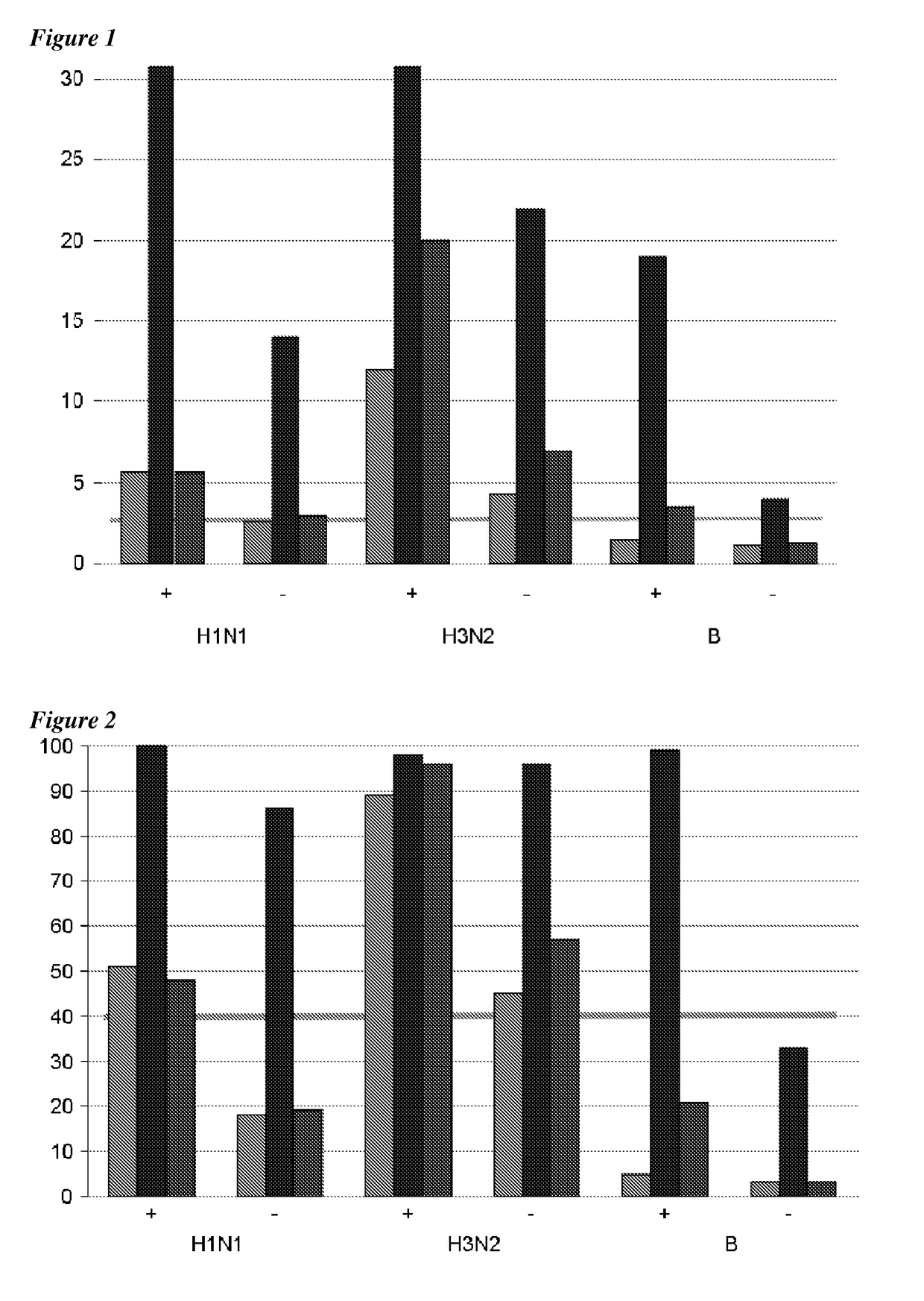
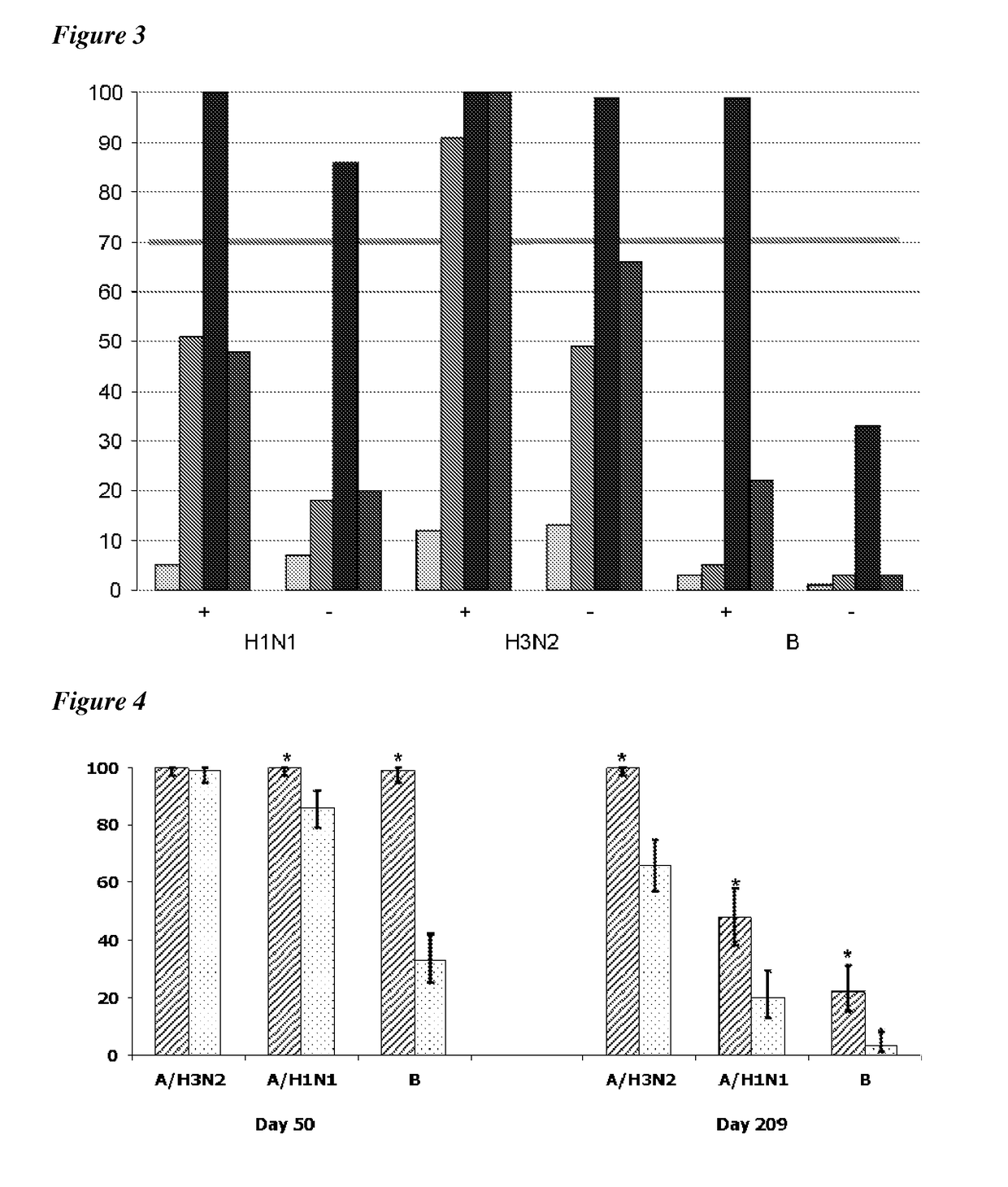
Adjuvanted influenza vaccines for pediatric use
ActiveUS8506966B2Enhance immune responseHigh seroprotection rateSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSeroconversion
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
Polynucleotide herpes virus vaccine
InactiveUS7094767B2Avoid infectionAmeliorate HSV-related diseaseSugar derivativesGenetic material ingredientsMammalSeroconversion
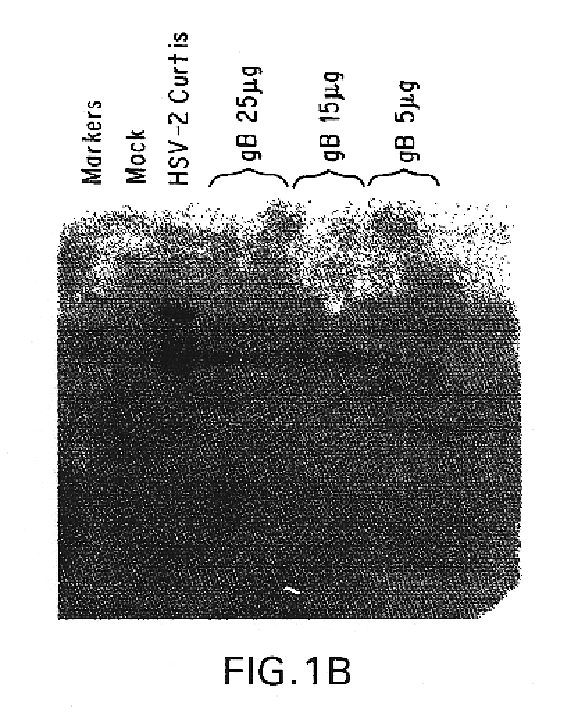
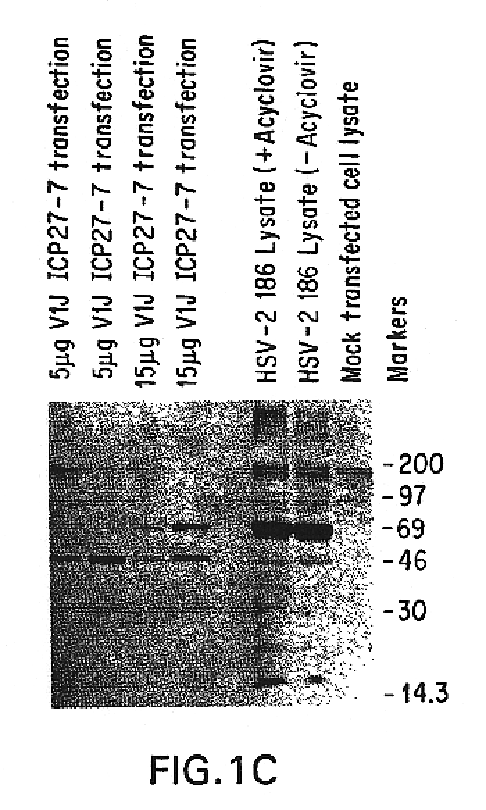
Genes encoding herpes simplex virus type 2 (HSV-2) proteins were cloned into eukaryotic expression vectors to express the encoded proteins in mammalian muscle cells in vivo. Animals were immunized by injection of these DNA constructs, termed polynucleotide vaccines or PNV, into their muscles. In a DNA titration, it was found that a single immunization of ≧0.5 μg of (one) PNV, gave >90% seroconversion by ten weeks post immunization. Immune antisera neutralized both HSV-2 and HSV-1 in cell culture. When animals were challenged with HSV-2, significant (p<0.001) protection from lethal infection was achieved following PNV vaccination. DNA constructs may be full-length, truncated and / or mutated forms and may be delivered along or in combination in order to optimize immunization and protection from HSV infection.
Owner:MERCK SHARP & DOHME CORP
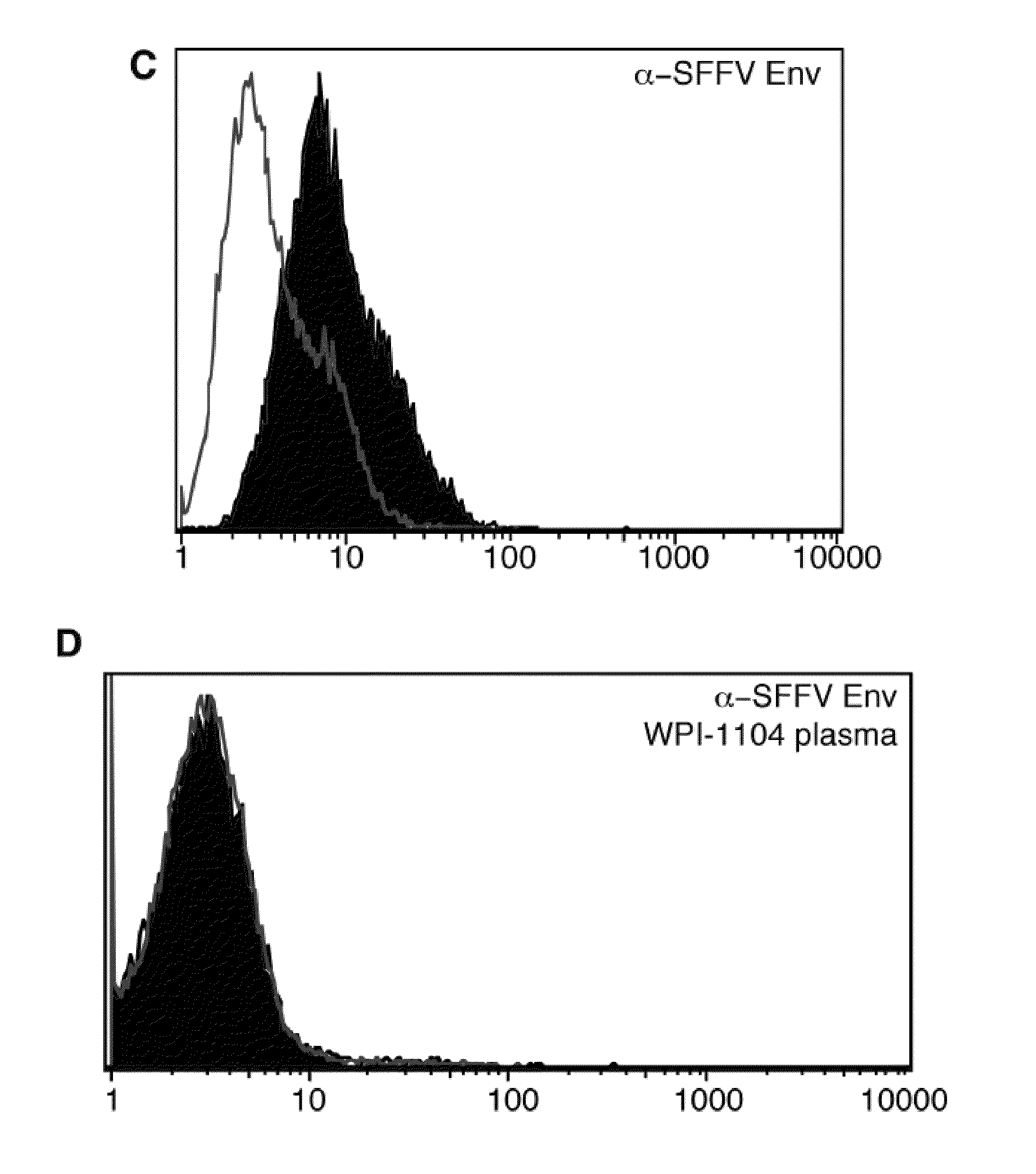
Seroconversion assays for detecting xenotropic murine leukemia virus-related virus
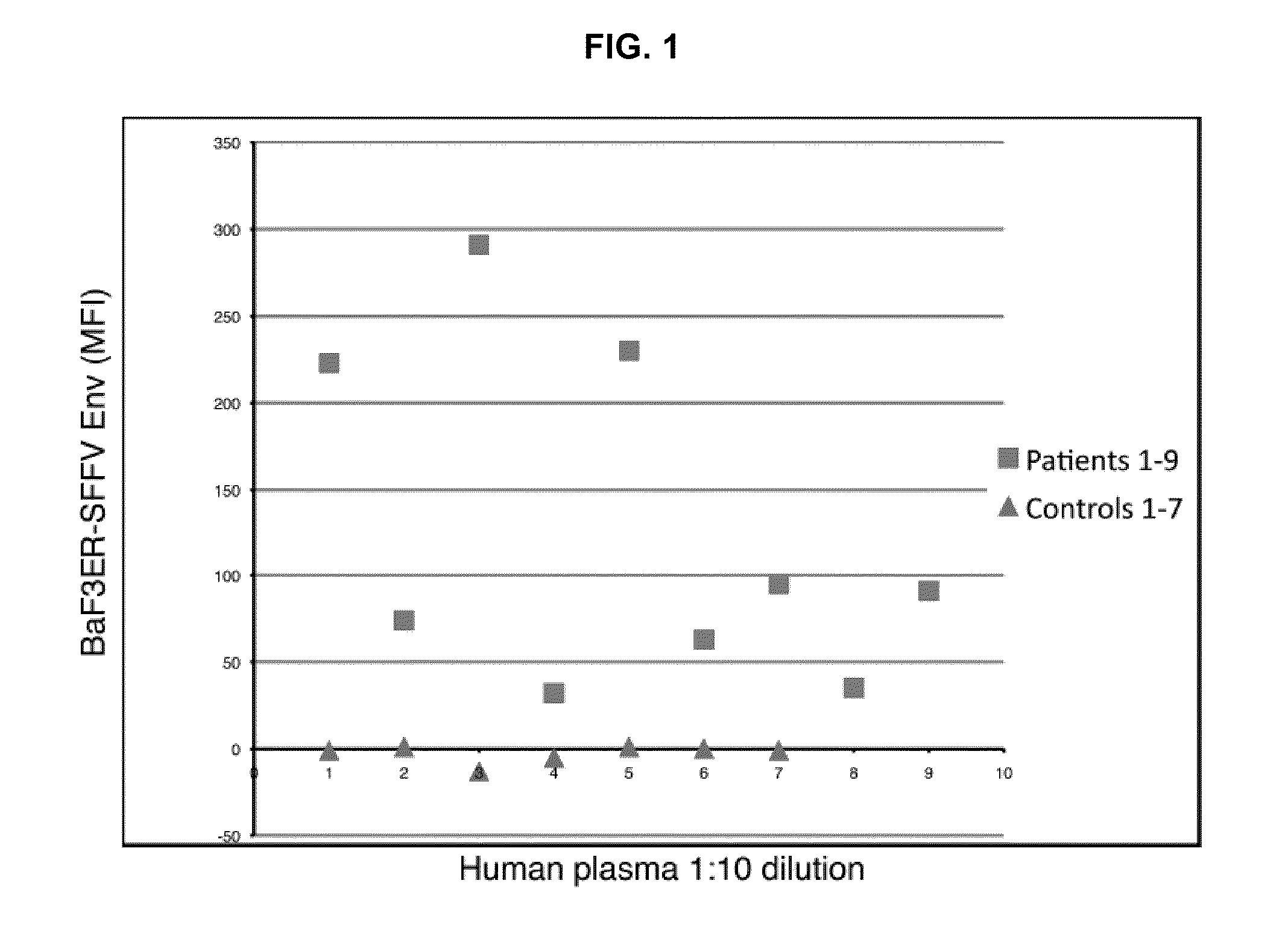
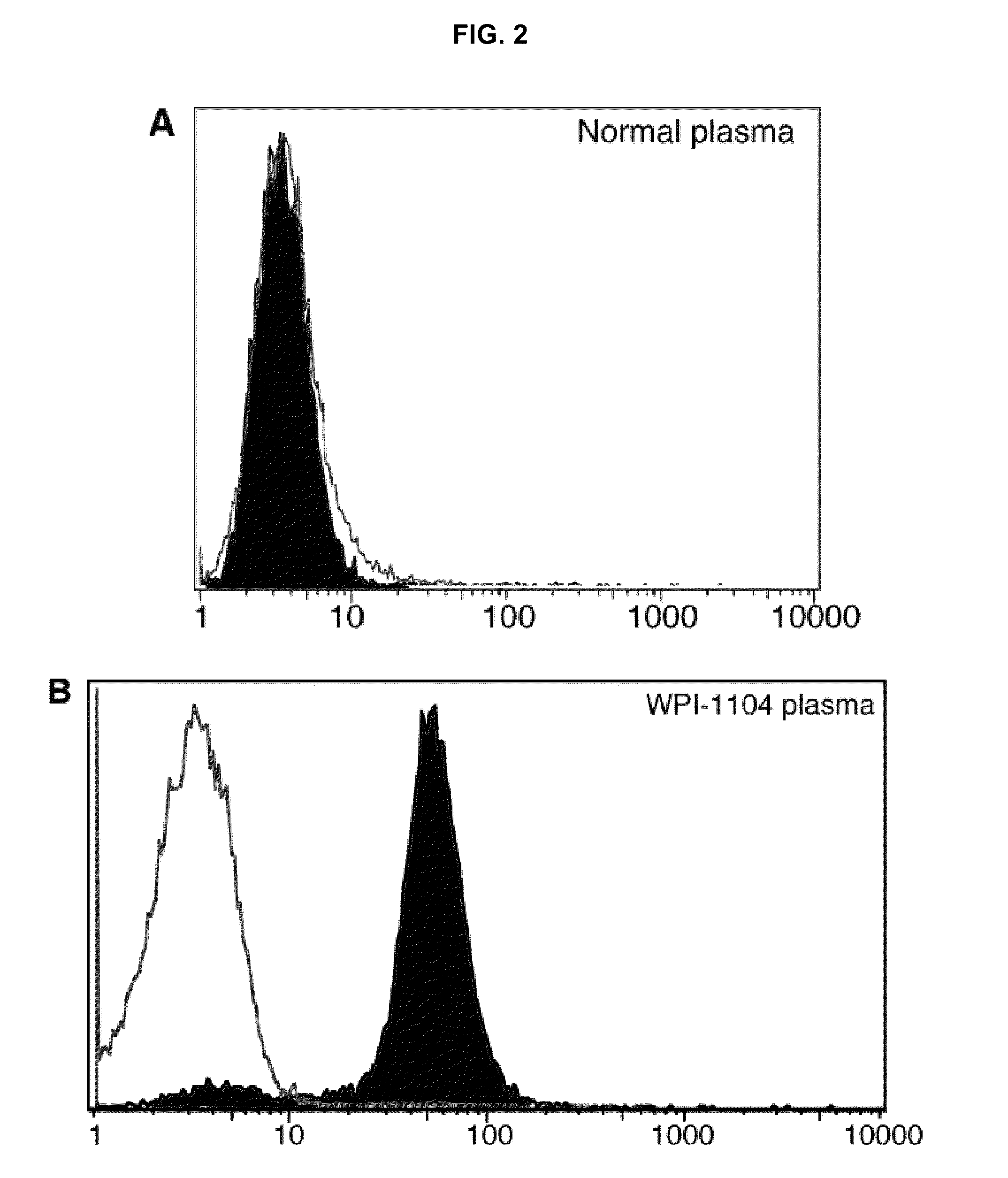
Methods of detecting, diagnosing, monitoring or managing an XMRV-related disease such as an XMRV-related neuroimmune disease such as chronic fatigue syndrome or an XMRV-related lymphoma such as mantle cell lymphoma in a subject are disclosed. These methods comprise determining presence, absence or quantity of antibodies against XMRV in a sample from a subject.
Owner:WHITTEMORE PETERSON INST FOR NEURO IMMUNE DISEASE
Adjuvanted influenza vaccines for pediatric use
ActiveUS20090220546A1Raising useful immune responseProvide immunitySsRNA viruses negative-senseViral antigen ingredientsSerum igeAdjuvant
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
Borrelia burgdorferi bacterial antigen diagnosic test using polymeric bait containing capture particles
InactiveUS20130085076A1Improve abilitiesReliable, rapid, inexpensive and non-invasiveLibrary screeningImmunoassaysDiagnostic testSeroconversion
The invention relates to both a sensitive method for the capture and detection of low-abundance Borrelia burgdorferi (Bb) bacterial antigens allowing for the diagnosis of Lyme Disease using standard immunoassays. Furthermore, this invention allows the antigen to be identified in a sample of urine, serum, or other biological fluids isolated from humans and animals. The invention provides a method to capture, concentrate, separate and specifically quantify the abundance of Bb antigens using immunoassays. The detection of Bb Outer Surface Protein A is presented as an example of the disclosed invention. High sensitivity levels, low cost and easily collected biofluids allow this technology to reach patients in clinics as well as POC applications for the early detection of Lyme disease prior to seroconversion. A kit containing necessary reagents and the method for diagnosis, monitoring or assessing lyme disease using an immunoassay such as an ELISA, western blot or RPPMA is disclosed.
Owner:GEORGE MASON INTPROP INC +1
Recombinant S1 protein of novel mutant strain of porcine epidemic diarrhea virus and subunit vaccine of recombinant S1 protein
ActiveCN104046637AGood reactogenicityAntiviralsAntibody medical ingredientsPseudomonas aeruginosa exotoxin ASeroconversion
The invention discloses a recombinant S1 protein of a novel mutant strain of a porcine epidemic diarrhea virus and a subunit vaccine of the recombinant S1 protein. The protein is obtained by the steps of cloning a fusion gene fragment PE(delta III)-S1(m)-KDEL3 containing a KDEL3 gene sequence, a gene sequence of III-region-deleted pseudomonas aeruginosa exotoxin A and an S1(m) gene sequence to a baculovirus vector to obtain a recombinant vector; carrying out recombinant vector site specificity transposition on a seroconversion DH10Bac competent cell of the recombinant vector to obtain a recombinant bacmid; transfecting the recombinant bacmid to an Sf9 cell under the mediation of a liposome to obtain a recombinant Sf9 cell; expressing by using the recombinant Sf9 cell. According to the invention, a PE(delta III)-S1(m)-KDEL3 fusion protein is firstly expressed by using a baculovirus expression system on the basis of optimizing the predilection of a codon of mammalian with S1 genes, so that the recombinant baculovirus for efficiently and accurately expressing the fusion protein is obtained. Proved by IFA and Western blot, the recombinant baculovirus can be used for accurately expressing the fusion protein, and the fusion protein has favorable reactionogenicity.
Owner:NANJING AGRICULTURAL UNIVERSITY
Intradermal influenza vaccine
InactiveUS20100136053A1Not provideNot meetSsRNA viruses negative-senseViral antigen ingredientsHemagglutininSeroconversion
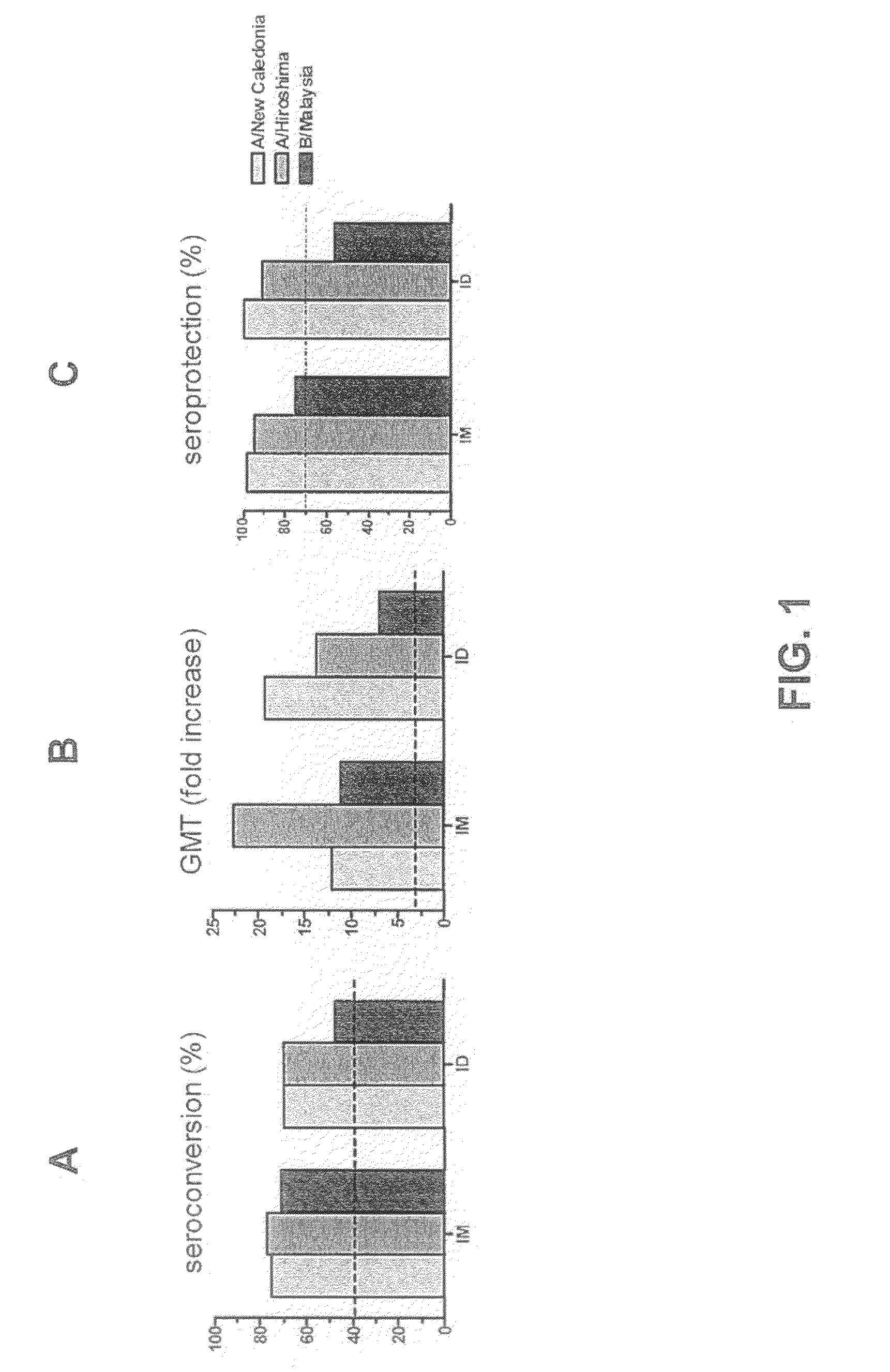
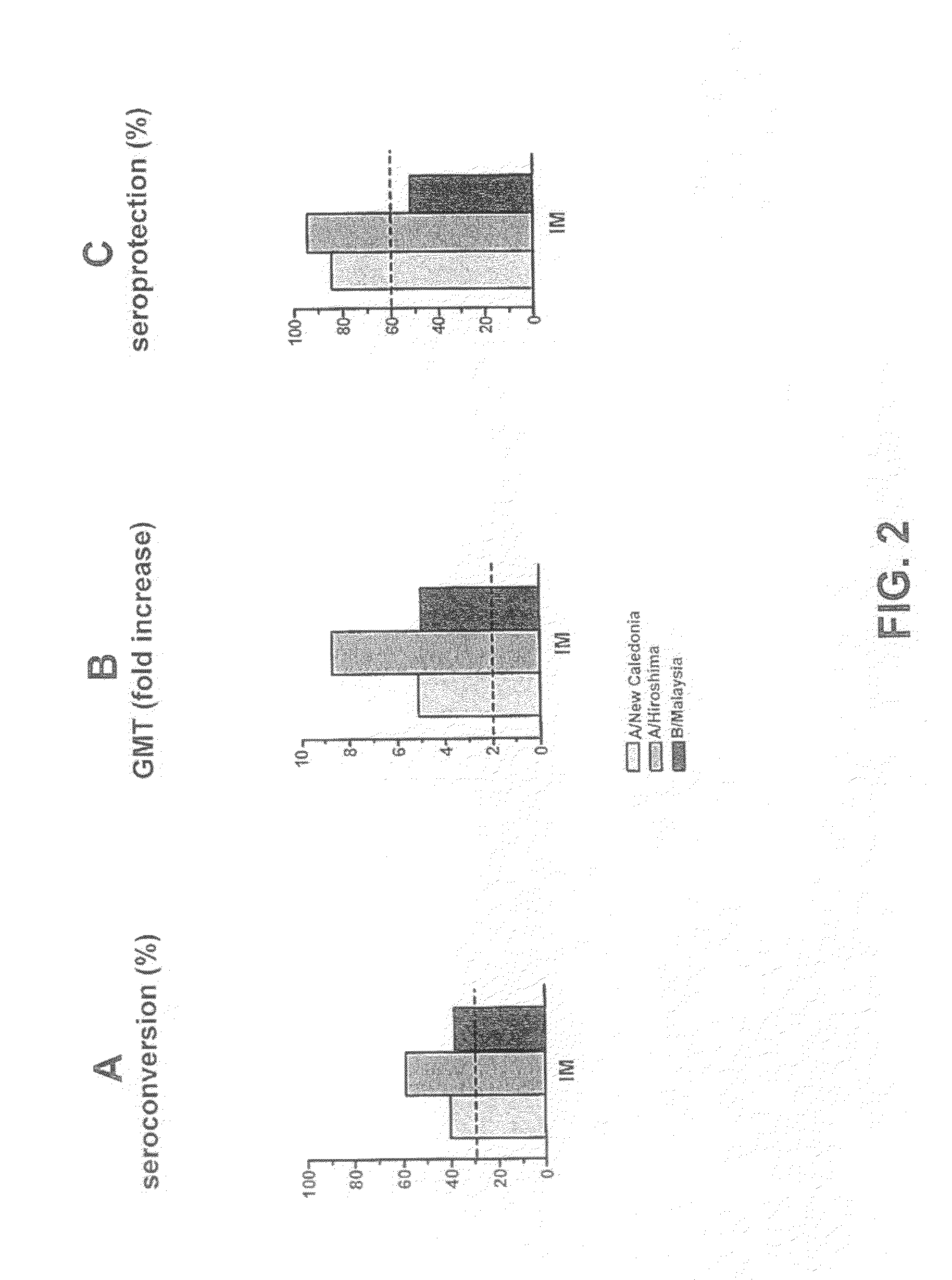
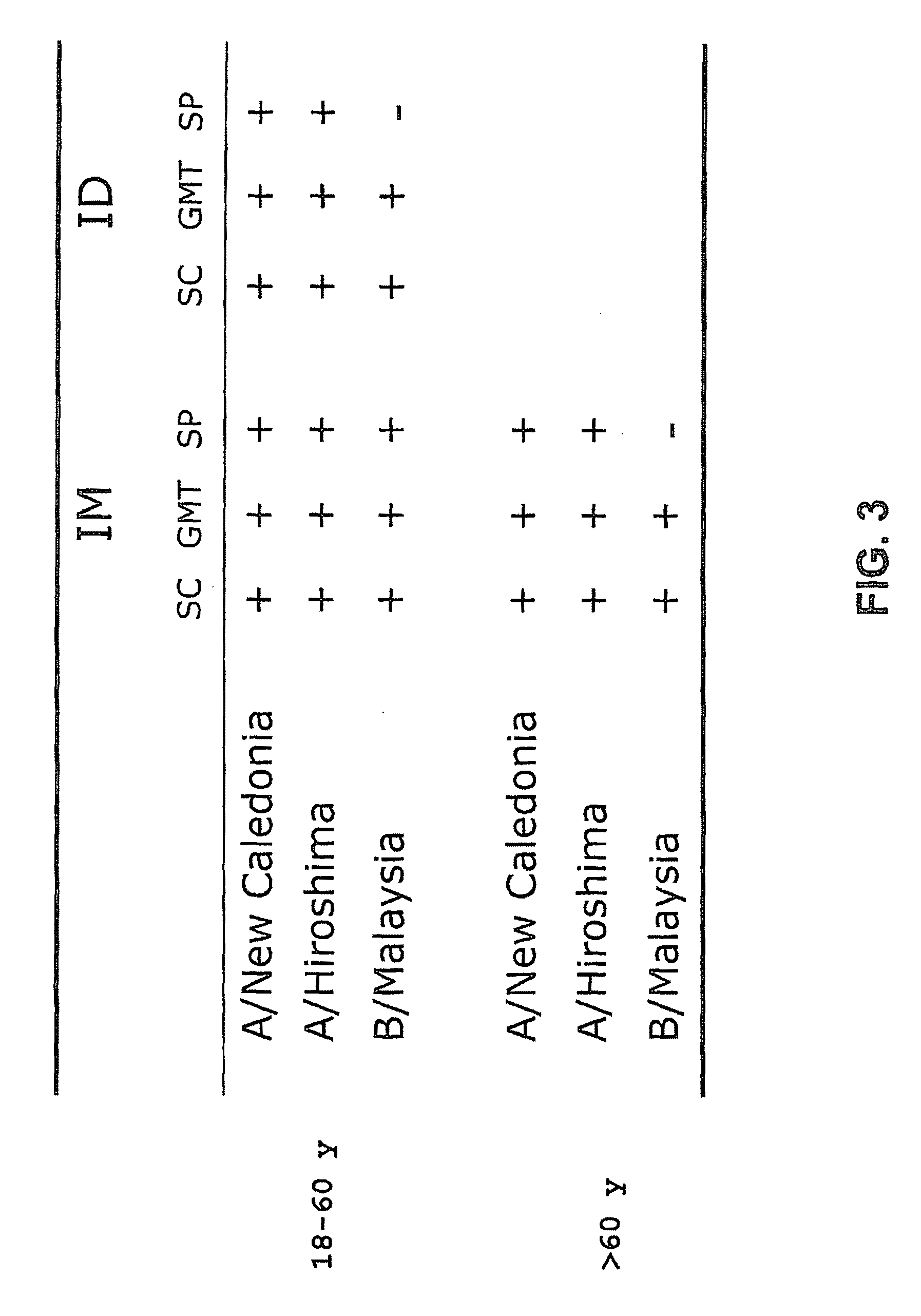
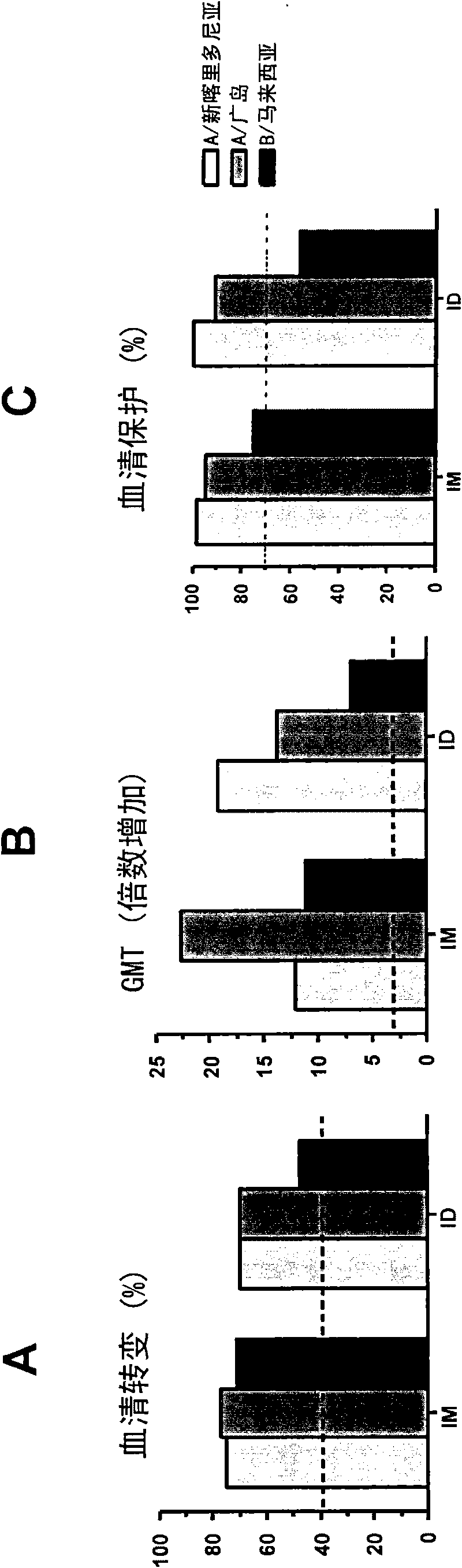
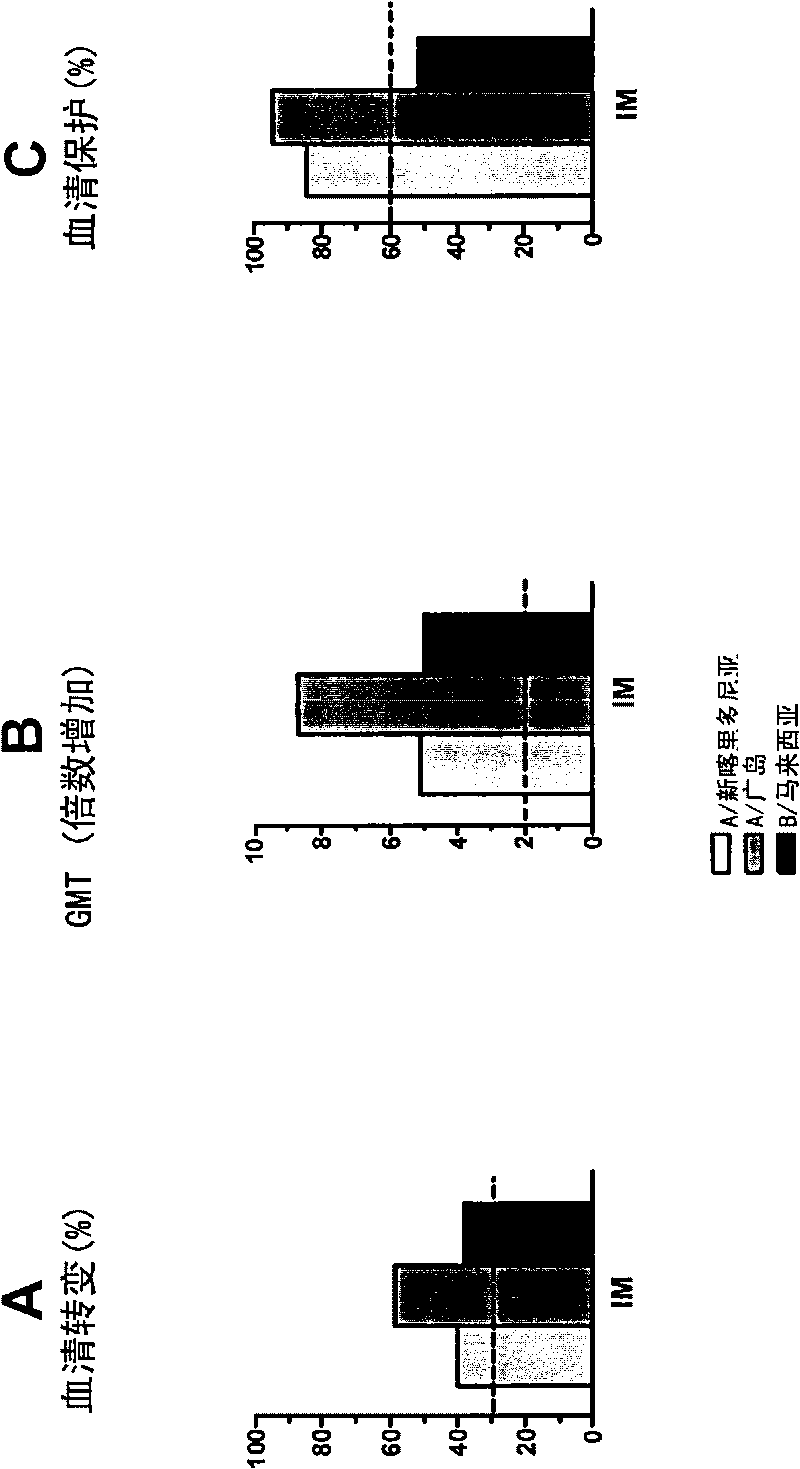
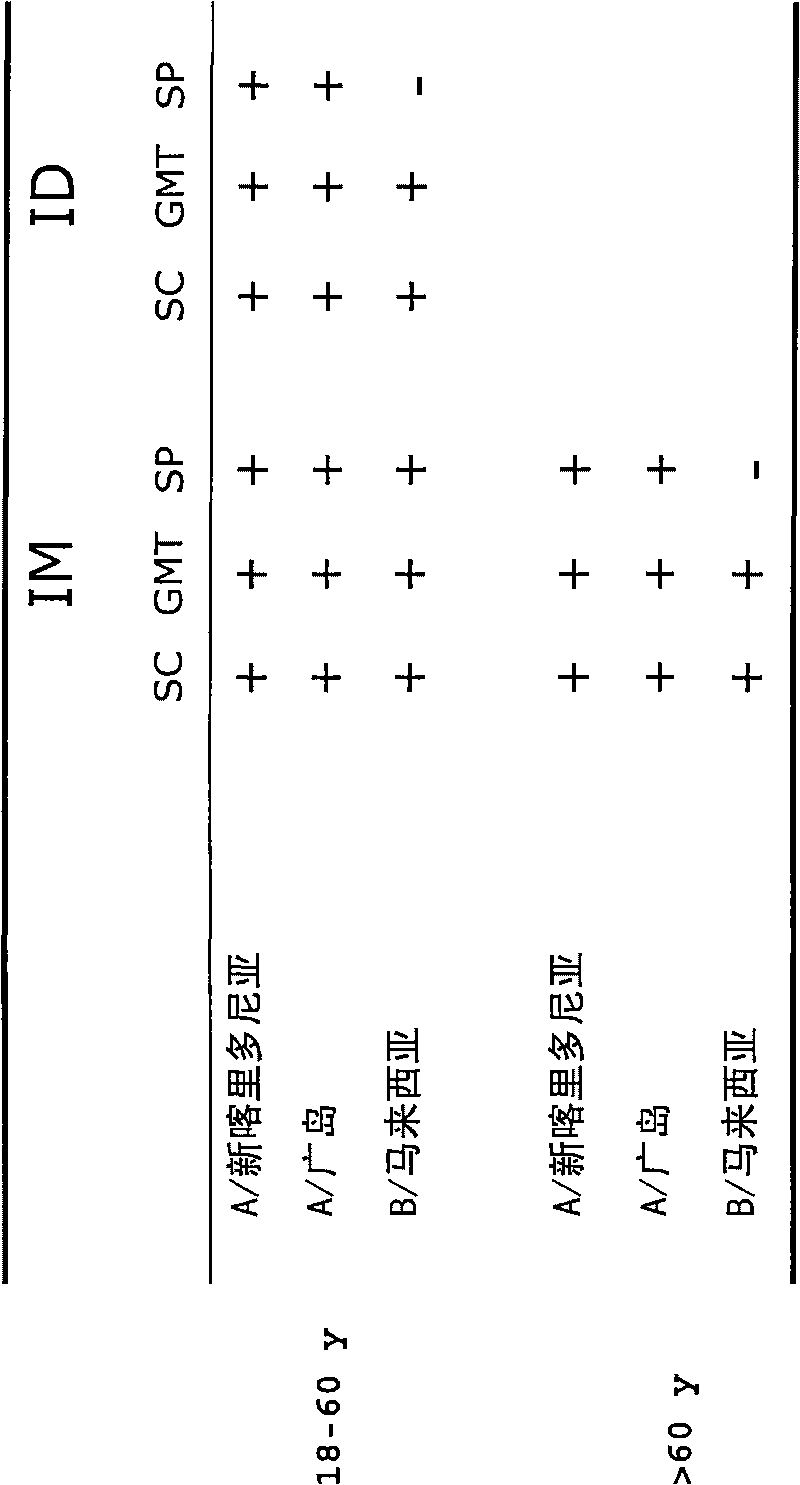
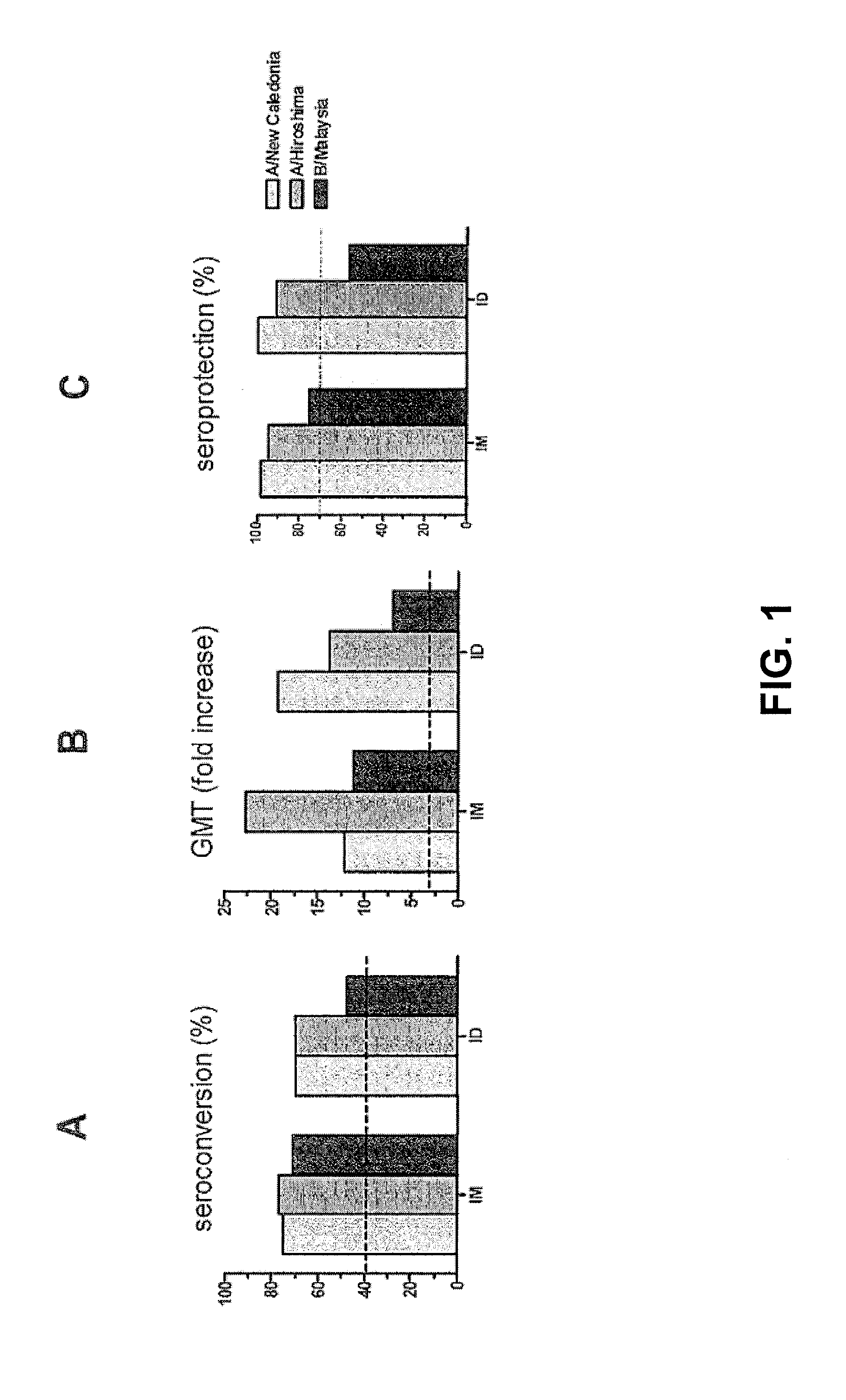
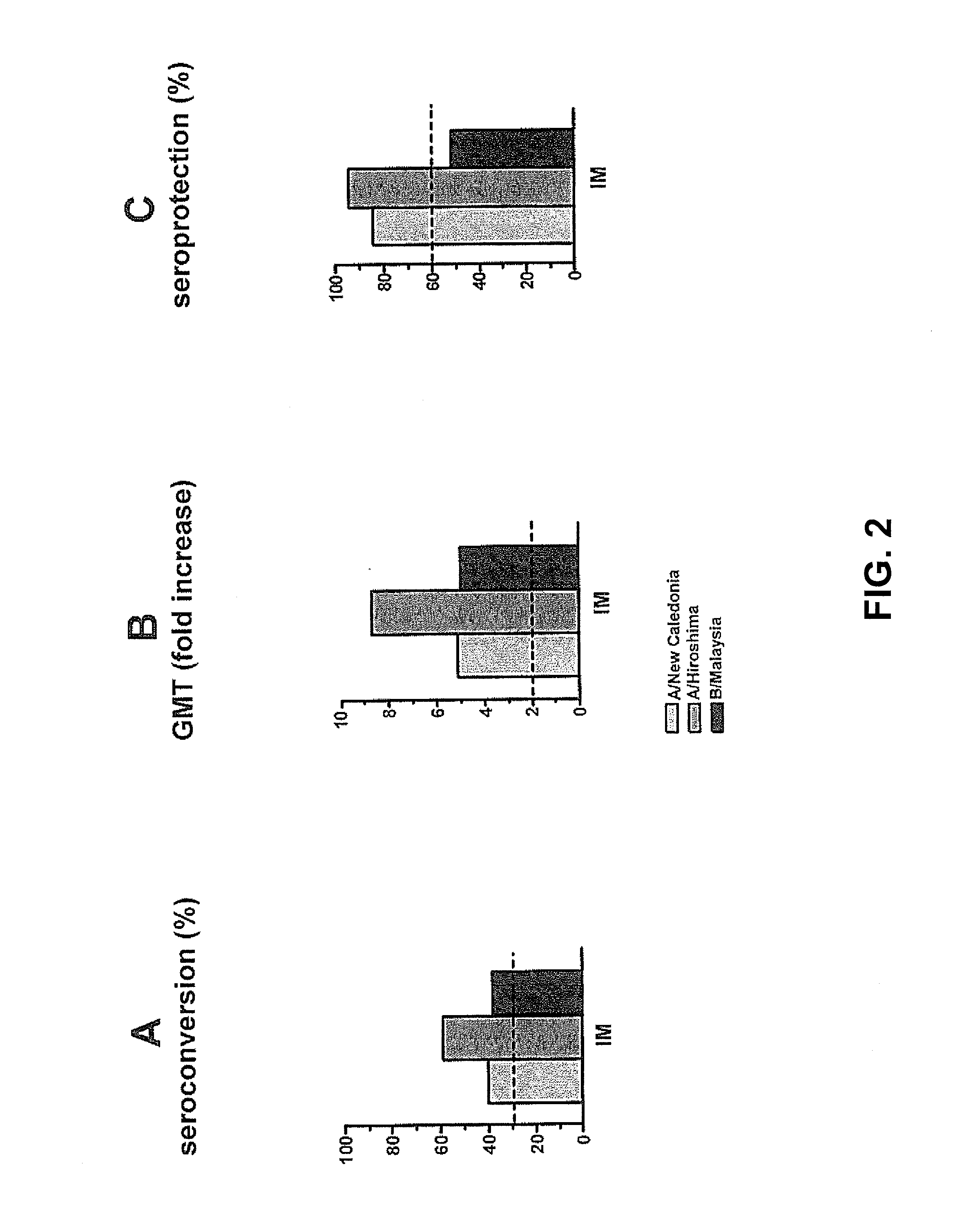
The invention relates to virosome-based influenza vaccines for the manufacture of medicaments that are administered intradermally in humans. The invention provides (trivalent) compositions comprising low doses of hemagglutinin (HA) antigen in a virosomal preparation that fulfill the immune response standards with respect to seroconversion rates, GMT-fold increase and protection rates, for use in vaccination set-ups.
Owner:CRUCELL SWITZERLAND AG
Method for detecting nanbv associated seroconversion
InactiveUS20090155772A1Peptide/protein ingredientsMicrobiological testing/measurementAntigenSerum ige
The present invention relates to recombinant expression vectors which express segments of deoxyribonucleic acid that encode recombinant HIV and HCV antigens. These recombinant expression vectors are transformed into host cells and used in a method to express large quantities of these antigens. The invention also provides compositions containing certain of the isolated antigens, diagnostic systems containing these antigens and methods of assaying body fluids to detect the presence of antibodies against the antigens of the invention.
Owner:F HOFFMAN LA ROCHE LTD +1
Adjuvanted influenza vaccines for pediatric use
InactiveUS20180207258A1Enhance immune responseHigh seroprotection rateSsRNA viruses negative-senseViral antigen ingredientsSerum igeAdjuvant
An influenza vaccine adjuvanted with a sub-micron oil-in-water emulsion elicits significantly higher immune responses in human pediatric populations. Compared to an existing unadjuvanted pediatric influenza vaccine, the adjuvanted vaccines provided herein can induce in children a longer persistence of high serum antibody titers and also longer seroconversion and seroprotection. The improvement in immune responses is seen for both influenza A virus and influenza B virus strains, but it is particularly marked for influenza B virus. Moreover, while the existing vaccine provides poor immunity in children after a single dose, the adjuvanted vaccine provides high seroprotection rates against the influenza A virus H3N2 subtype even after a single dose. Furthermore, the adjuvanted vaccine offers significantly better seroprotection against mismatched strains of influenza A virus.
Owner:SEQIRUS UK LTD
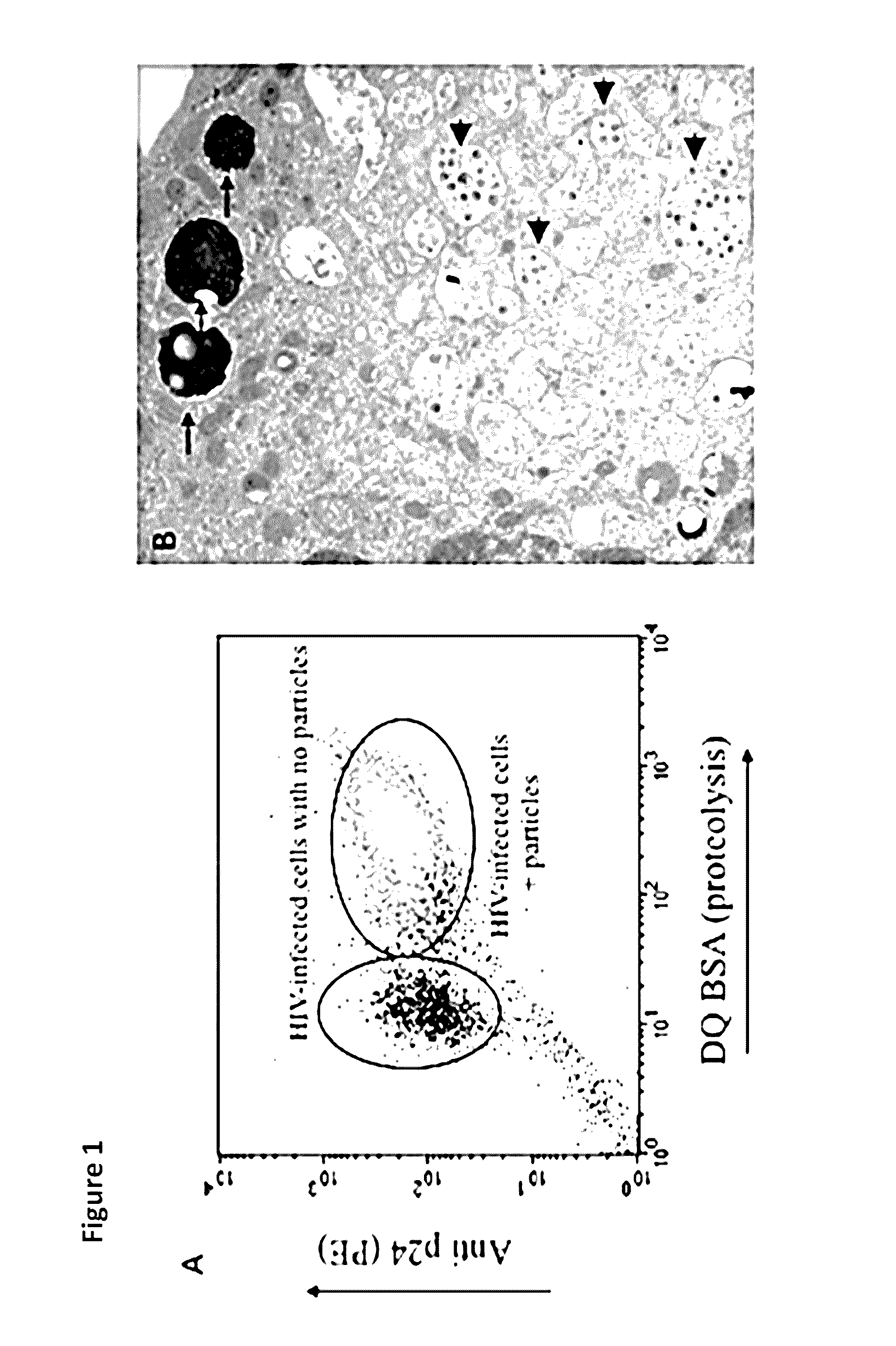
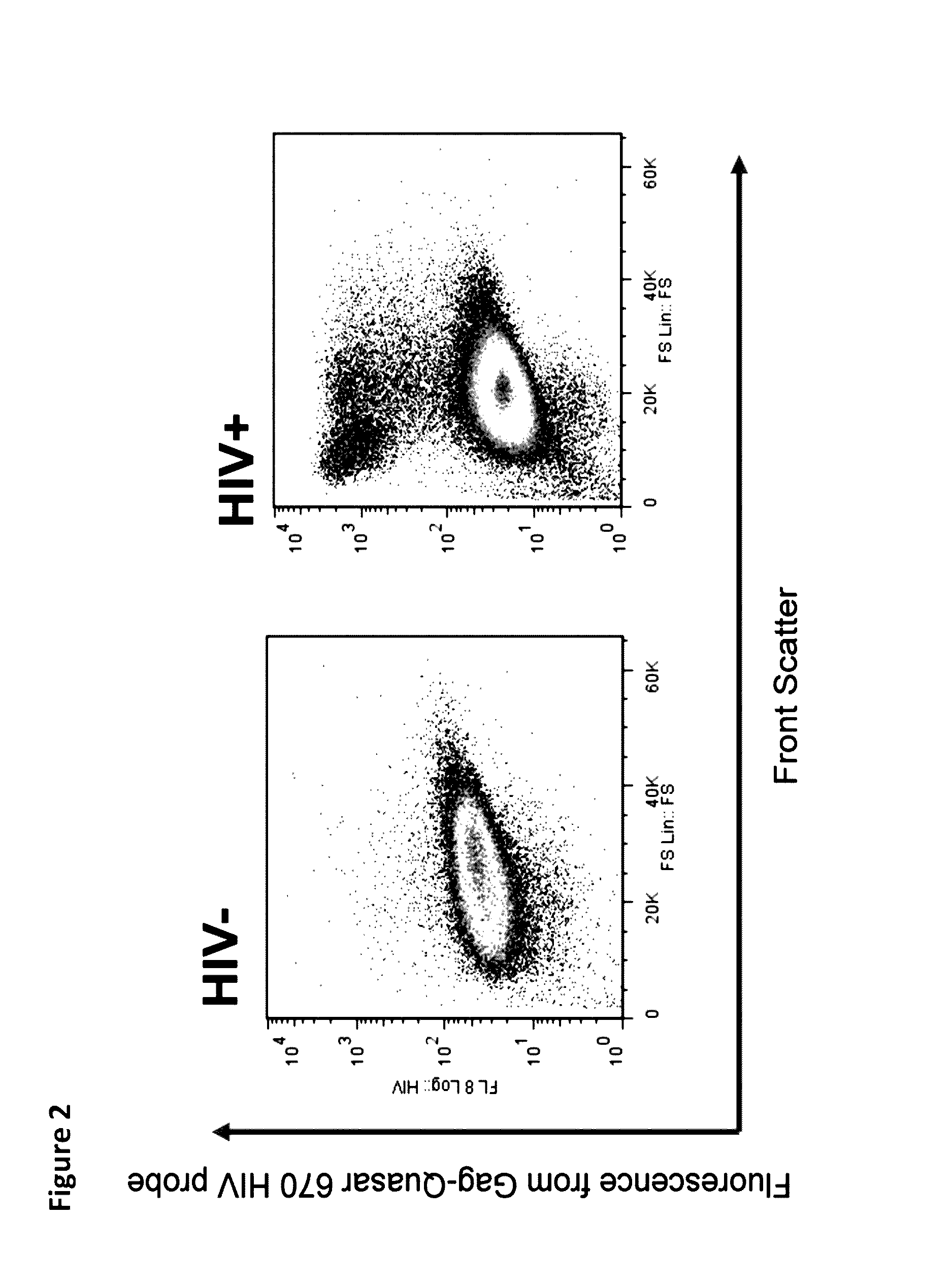
Methods for diagnosing human immunodeficiency virus infections
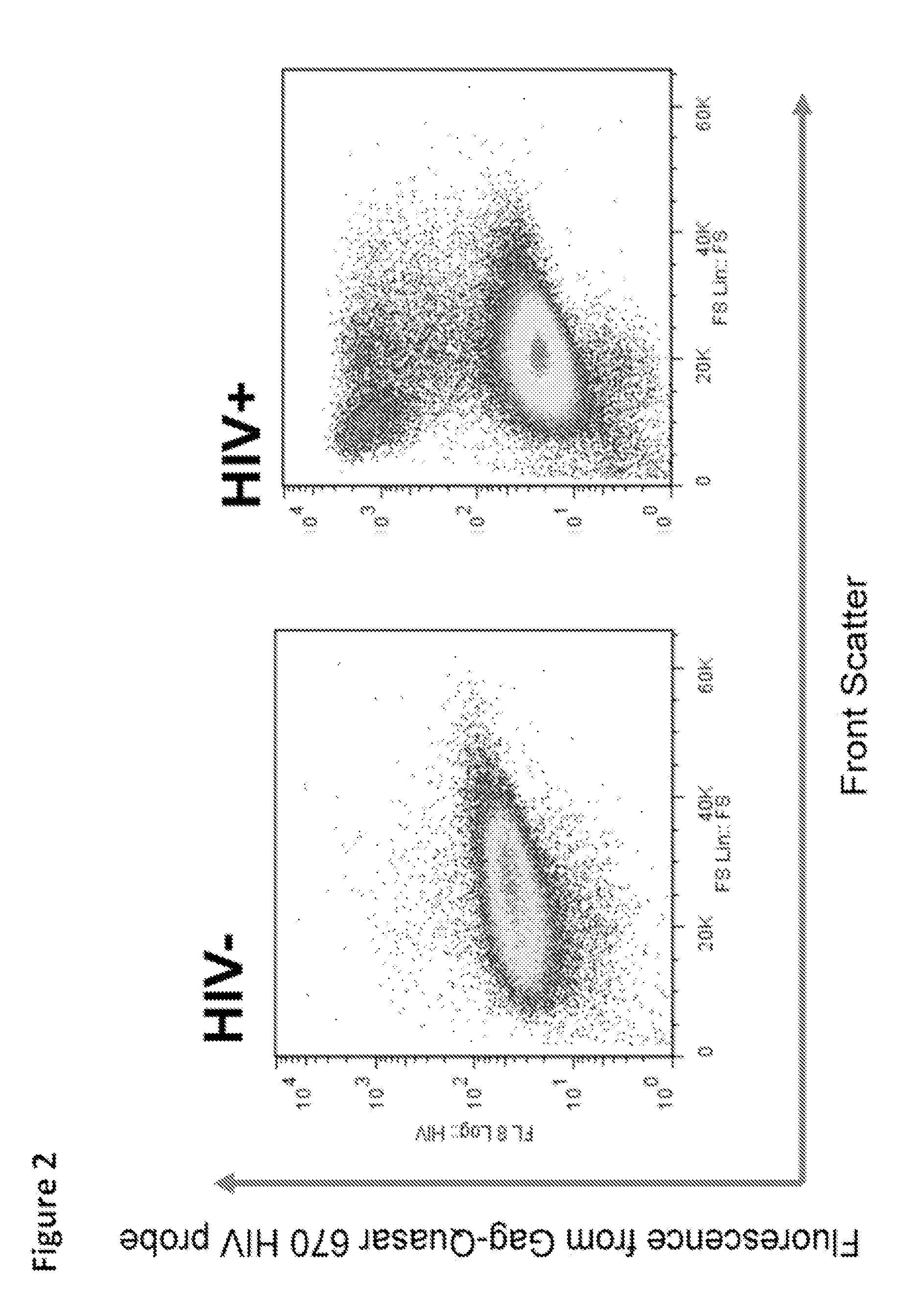
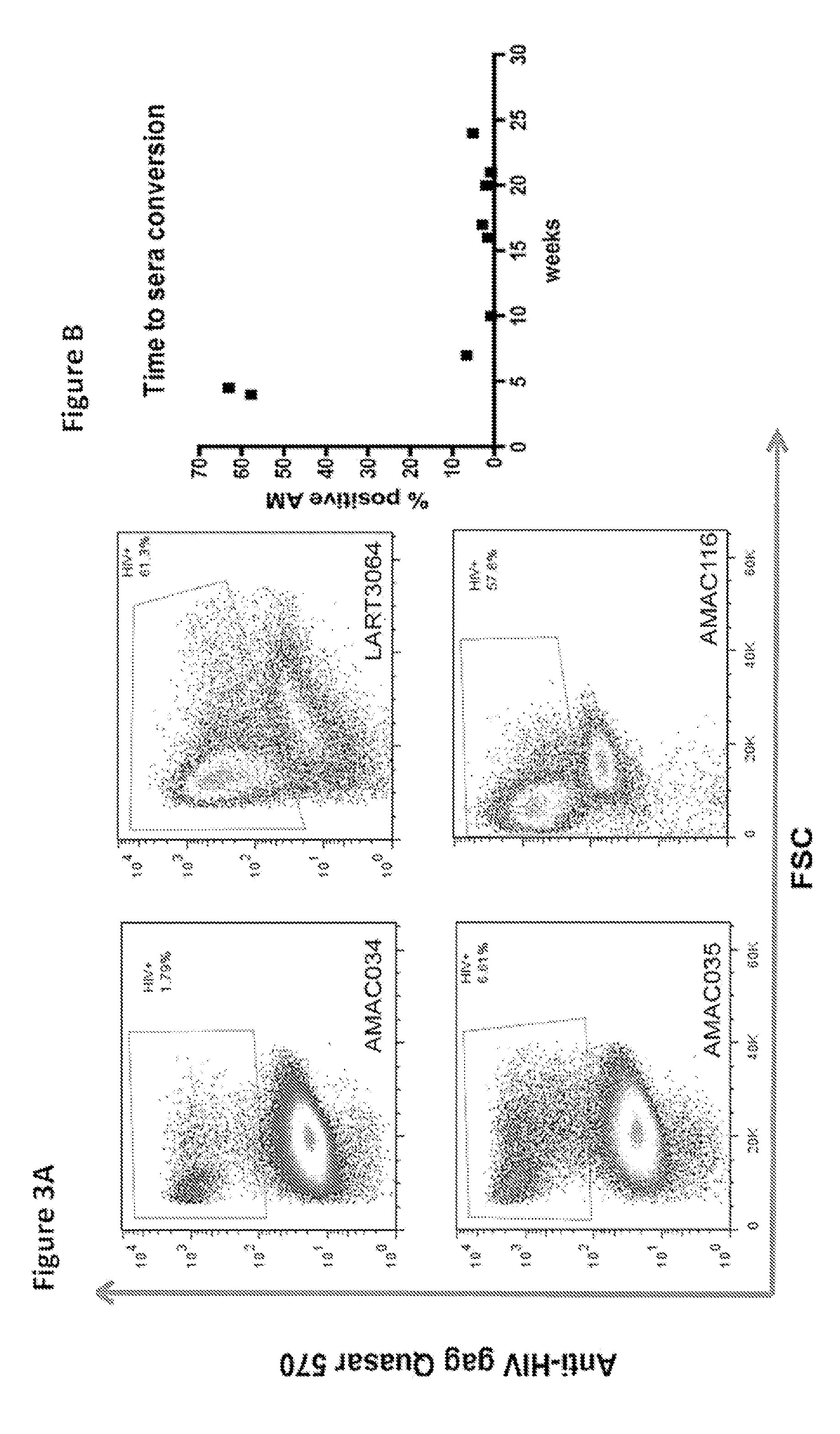
ActiveUS20140342934A1Microbiological testing/measurementLibrary screeningSeroconversionViral infection
Provided is a method for identifying human immunodeficiency virus (HIV) in a sample. The invention involves use of a plurality of fluorescently labeled oligonucleotide probes and flow cytometry to detect the presence or absence of HIV in macrophages that are obtained from a mucosal surface. The invention is particularly useful for detecting HIV infection prior to seroconversion.
Owner:CORNELL UNIVERSITY +1
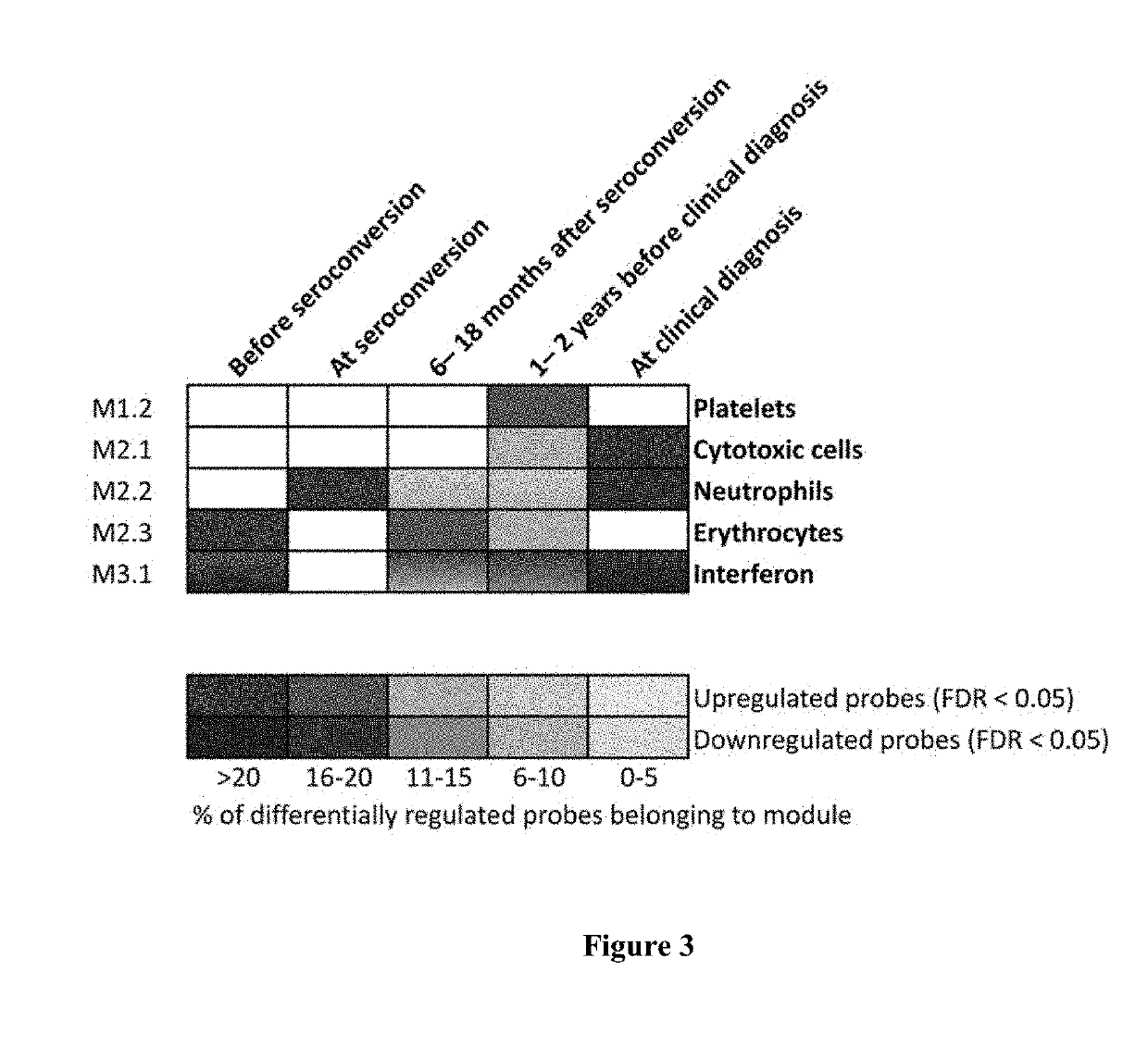
Method of Predicting Risk for Type 1 Diabetes
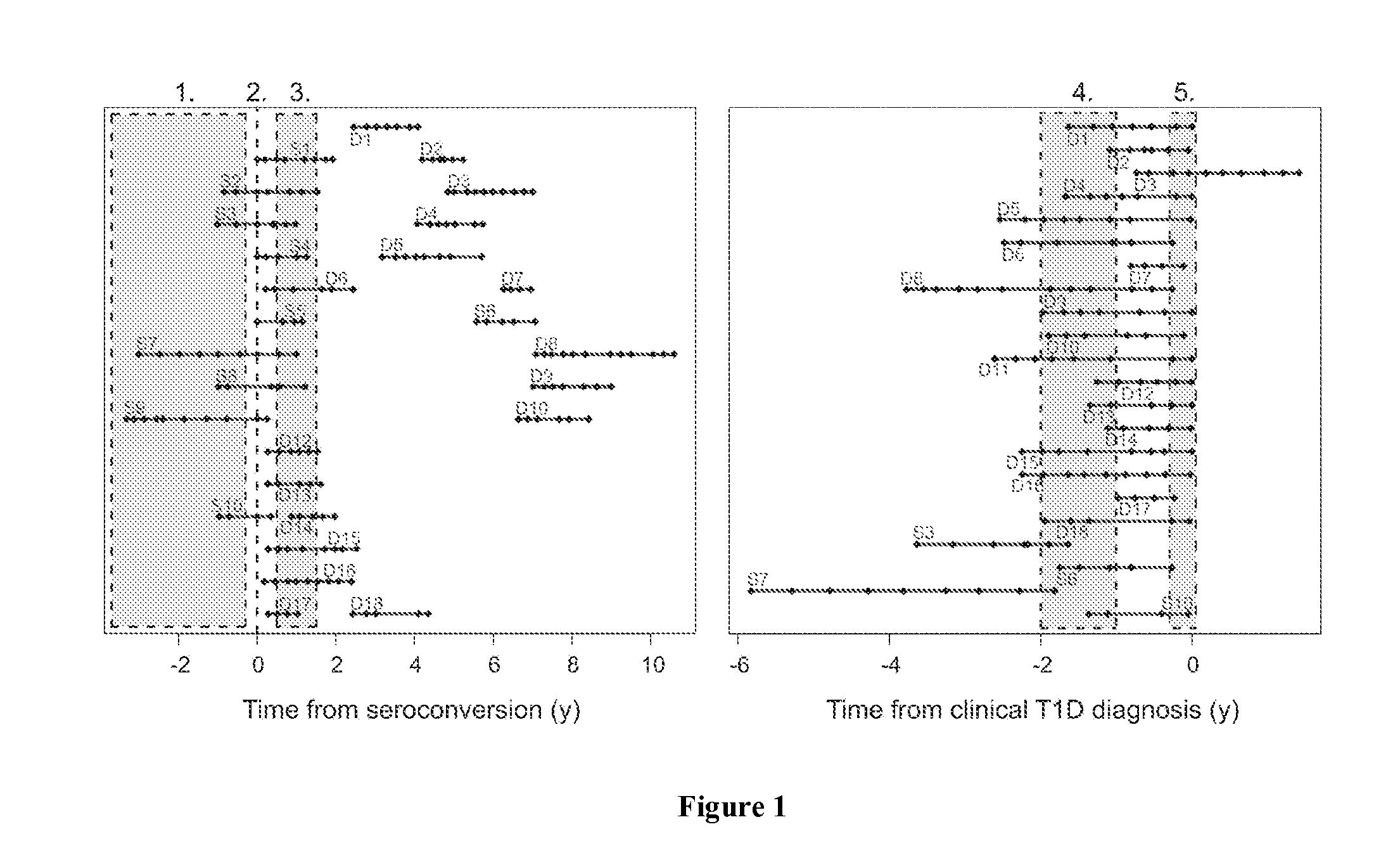
InactiveUS20160145687A1Sugar derivativesMicrobiological testing/measurementSeroconversionType 1 diabetes
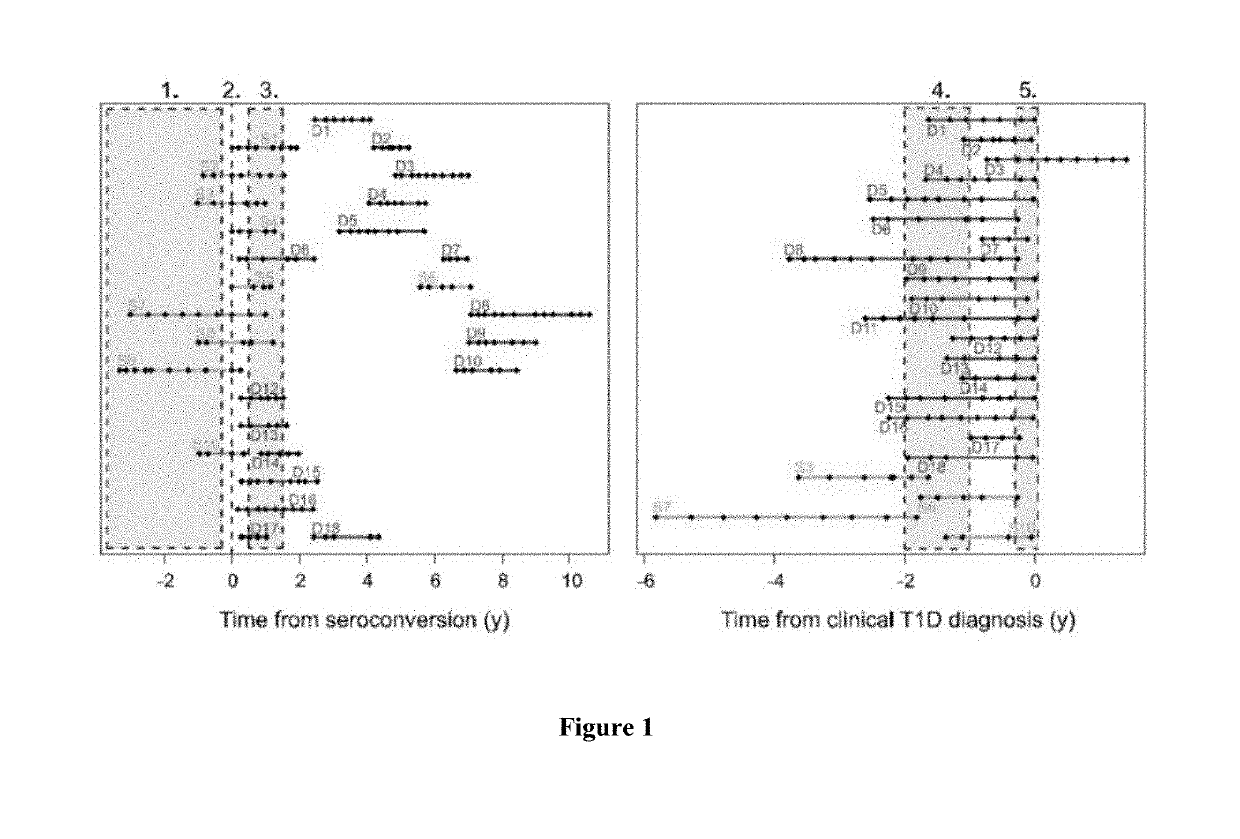
The present invention relates to methods, transcriptome profiles and kits useful for determining, before seroconversion, the risk that an individual will develop Type 1 diabetes (T1D).
Owner:TURUN YLIOPISTO
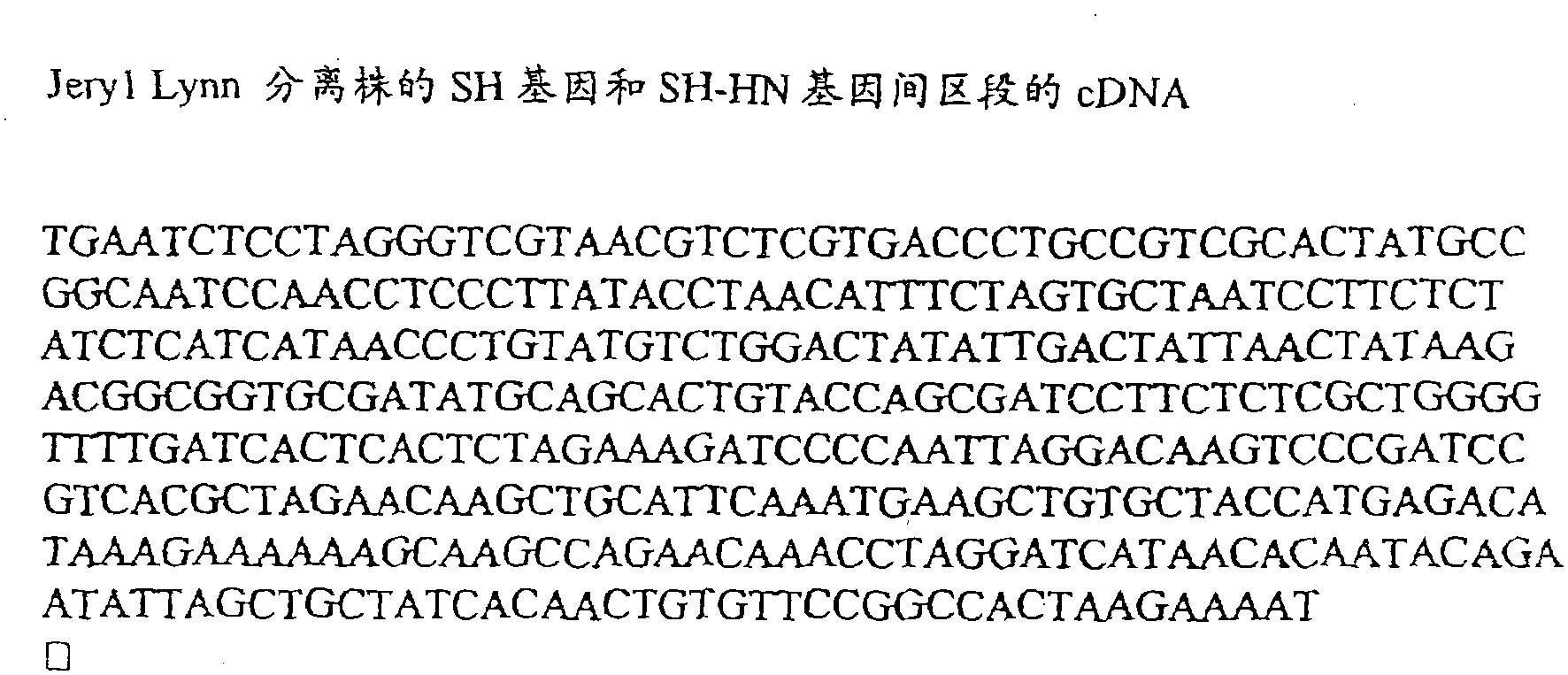
Vaccine against mumps containing a JERYL-LYNN virus strain
InactiveCN1763181AProtective responseCompensate for interferenceViruses/bacteriophagesAntibody medical ingredientsSerum igeJeryl Lynn
The present invention proposes a novel mumps vaccine comprising a homogeneously purified isolate derived from the Jeryl-Lynn strain of mumps virus. The vaccine in the preferred embodiment of the invention produces higher seroconversion rates and antibody titers than known commercial vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Methods of diagnosing viral infection
ActiveUS20200010898A1Well formedMicrobiological testing/measurementMaterial analysisAntigenHepatitis B immunization
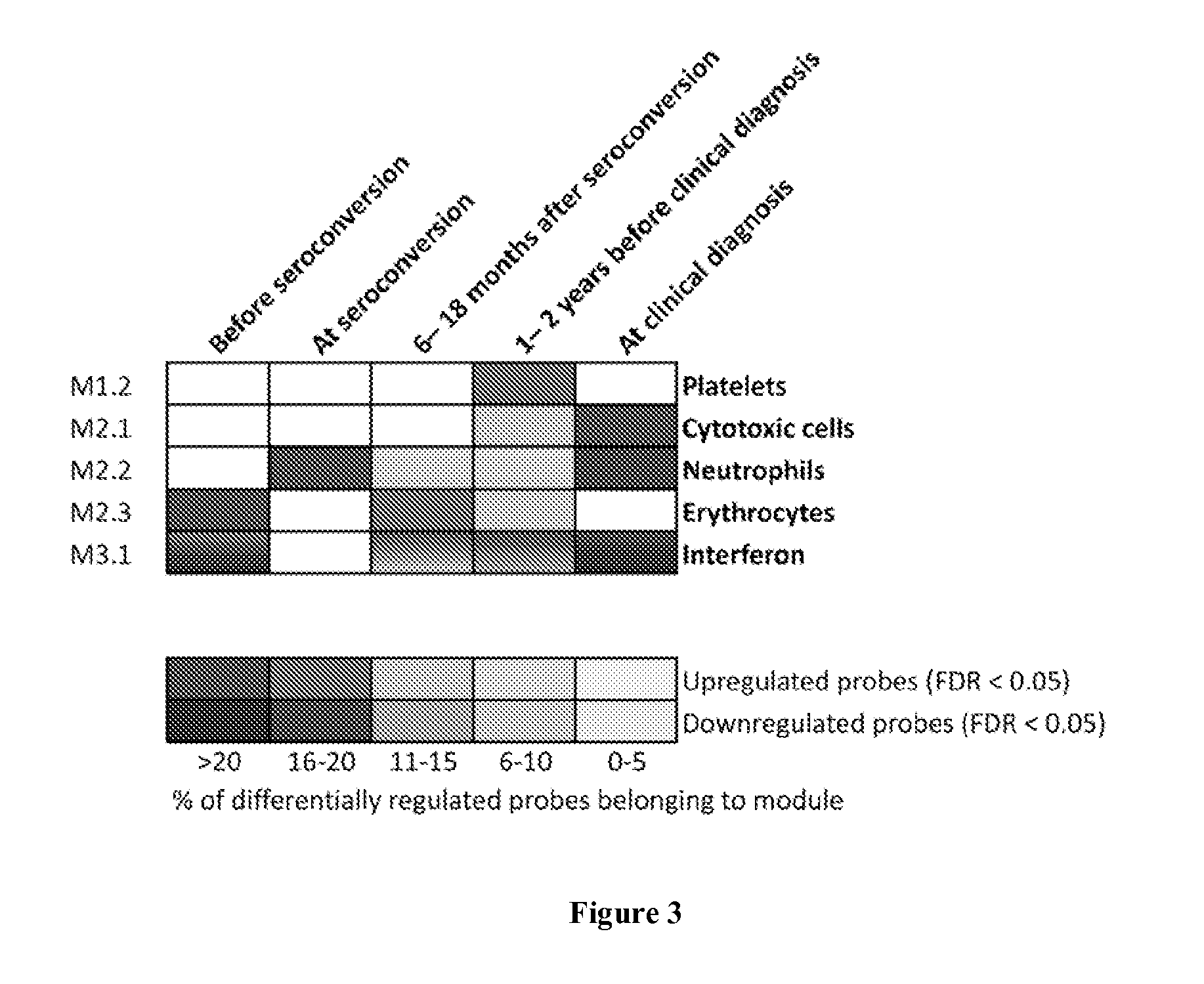
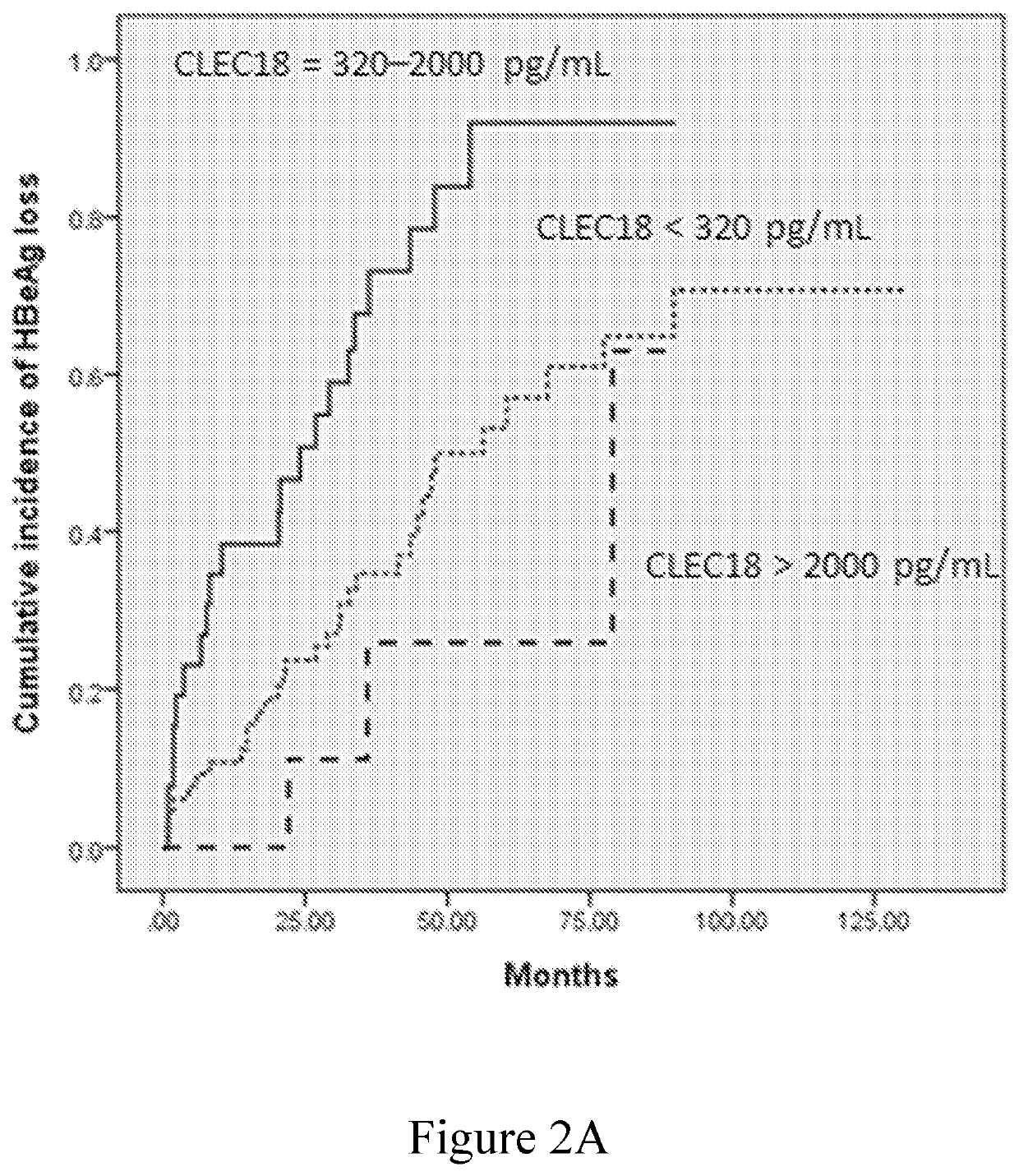
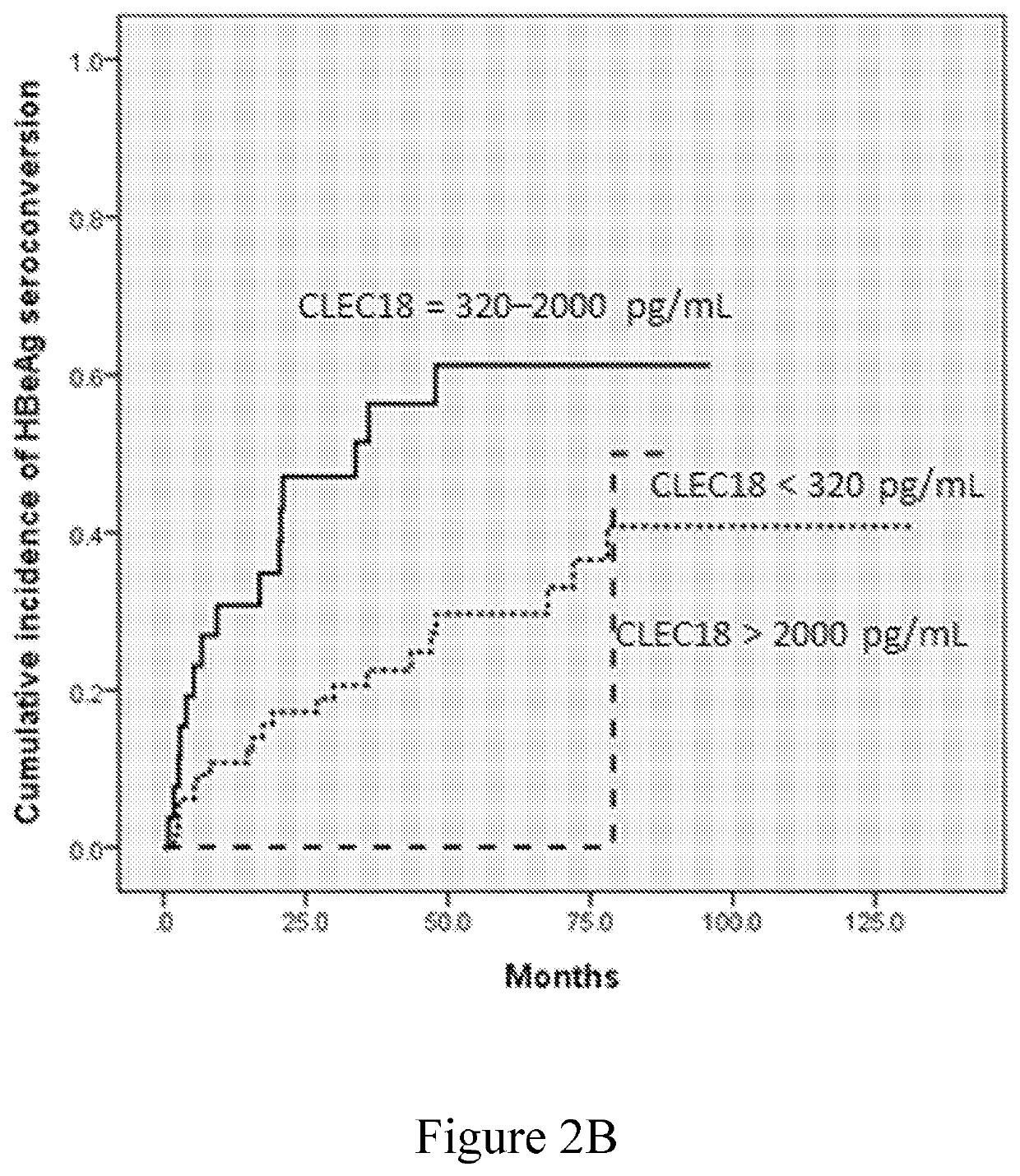
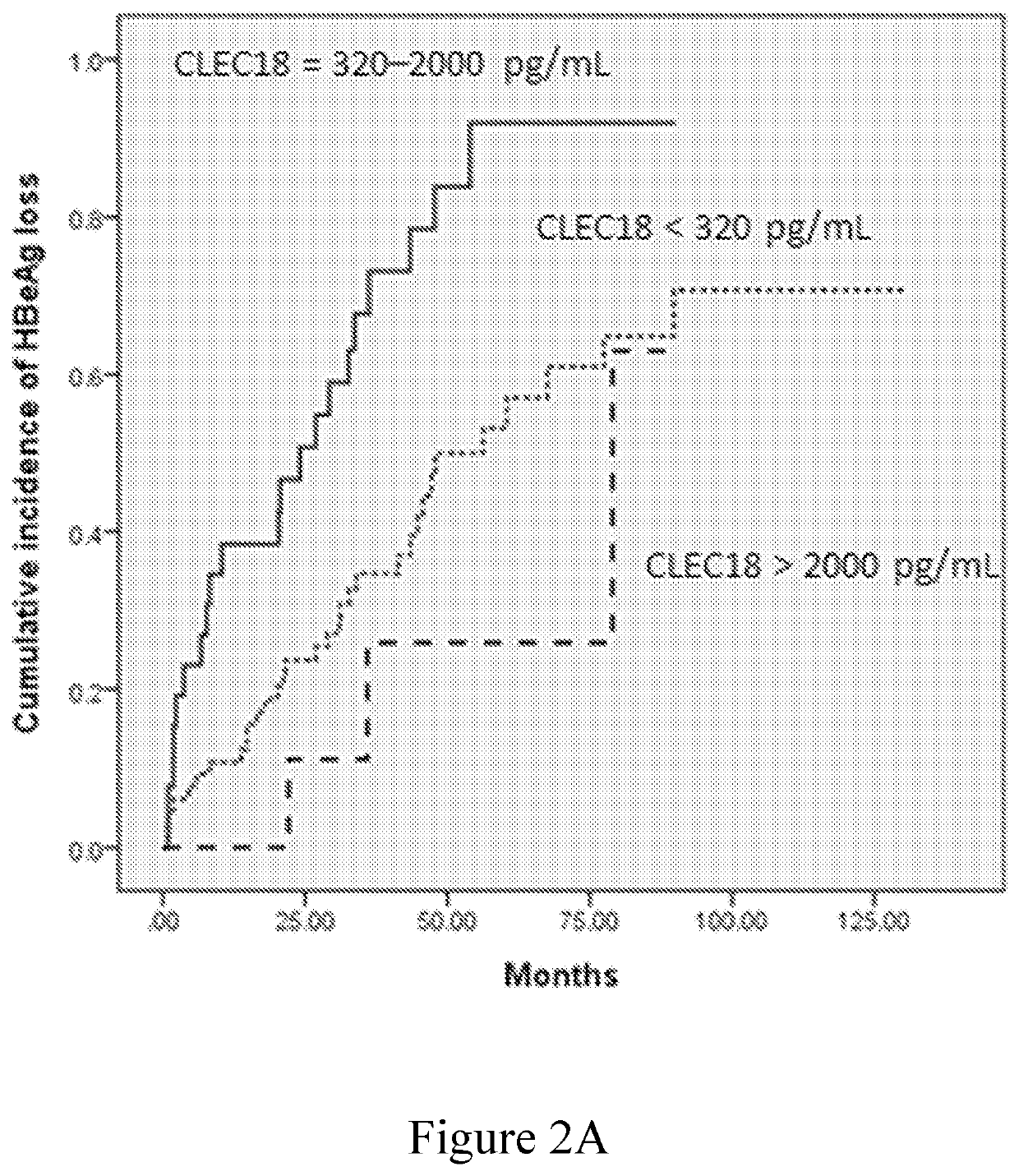
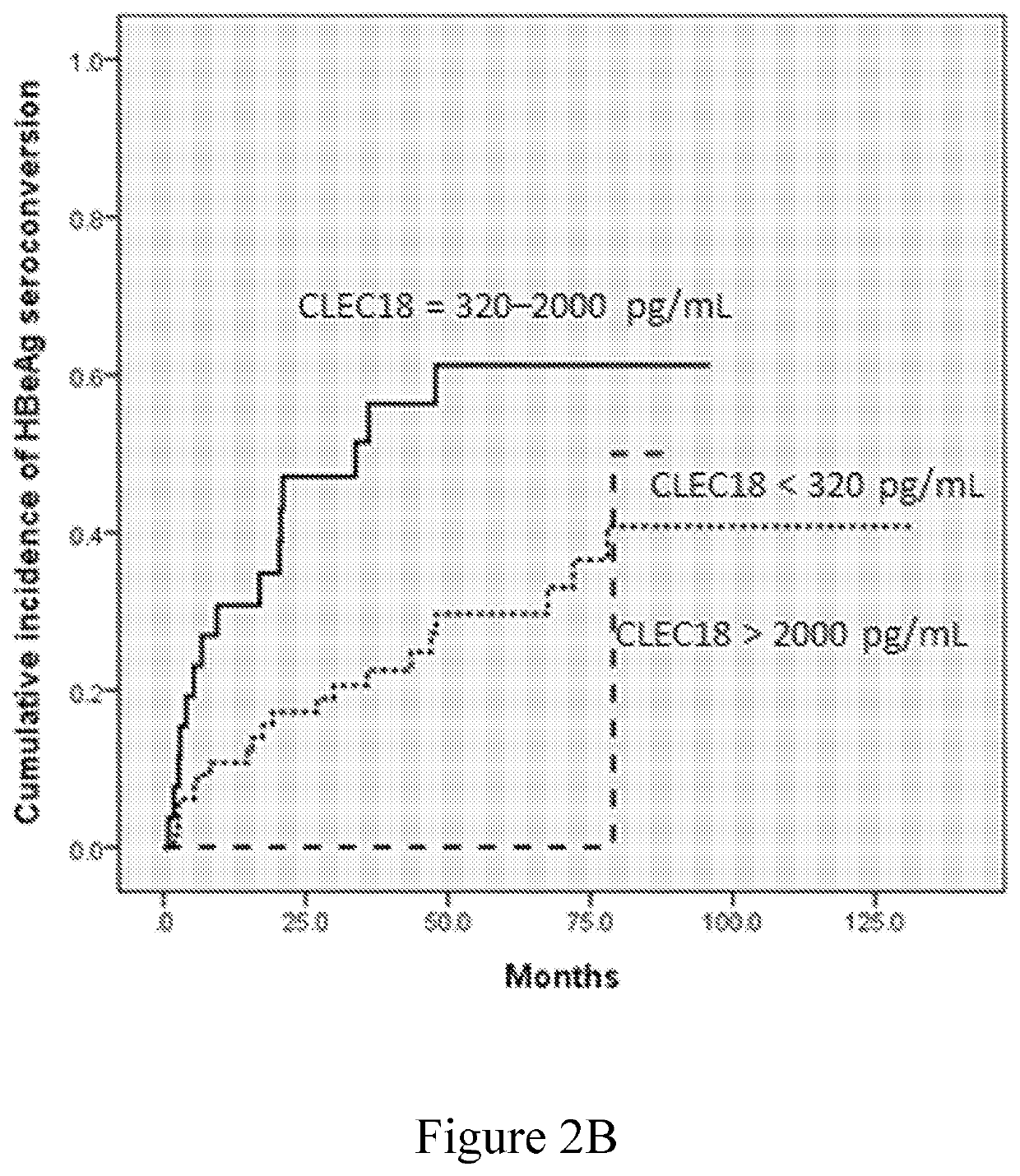
Disclosed herein is a novel use of C-type lectin 18 (CLEC18) in disease prognosis. According to embodiments of the present disclosure, the mRNA or protein level of CLEC18 may serve as an indicator for diagnosing hepatitis B virus (HBV) infection, hepatitis B e antigen (HBeAg) loss and seroconversion, and / or liver fibrosis.
Owner:ACAD SINIC
Hepatitis B virus resistant traditional Chinese medicine composition and preparation method thereof
InactiveCN102178789BPromote the productionPrevent liver fibrosisDigestive systemAntiviralsSeroconversionRadix Astragali seu Hedysari
The invention relates to the field of traditional Chinese medicine, in particular to a hepatitis B virus resistant traditional Chinese medicine composition composed of the following concrete components in parts: 15-20 parts of radix astragali, 12-15 parts of bupleurum, 12-15 parts of phellodendron bark, 6-12 parts of picrorhiza rhizome, 15-20 parts of giant knotweed, 15-20 parts of dyers woad leaf, 15-20 parts of asket fern, 12-15 parts of white atractylodes rhizome, 15-20 parts of tuckahoe, 15-20 parts of oldenlandia diffusa, 15-20 parts of red sage root and 5-8 parts of liquorice. The hepatitis B virus resistant traditional Chinese medicine composition can be prepared into capsules, decoction or extract, has the effects of clearing away heat and toxic material, strengthening the spleen and removing dampness through diuresis, has very strong efficacies of resisting hepatitis B virus, reducing HBVDNA (Hepatitis B Virus Deoxyribose Nucleic Acid) and promoting seroconversion of HBsAg (Hepatitis B Surface Antigen), and has multiple effects of protecting the liver, preventing pathological changes and the like.
Owner:刘明述
Intradermal influenza vaccine
The invention relates to virosome-based influenza vaccines for the manufacture of medicaments that are administered intradermally in humans. The invention provides (trivalent) compositions comprising low doses of hemagglutinin (HA) antigen in a virosomal preparation that fulfill the immune response standards with respect to seroconversion rates, GMT-fold increase and protection rates, for use in vaccination set-ups.
Owner:CRUCELL SWITZERLAND AG
A recombinant s1 protein and its subunit vaccine of a new mutant strain of porcine epidemic diarrhea virus
ActiveCN104046637BGood reactogenicityAntiviralsAntibody medical ingredientsPseudomonas aeruginosa exotoxin ASeroconversion
The invention discloses a recombinant S1 protein of a novel mutant strain of a porcine epidemic diarrhea virus and a subunit vaccine of the recombinant S1 protein. The protein is obtained by the steps of cloning a fusion gene fragment PE(delta III)-S1(m)-KDEL3 containing a KDEL3 gene sequence, a gene sequence of III-region-deleted pseudomonas aeruginosa exotoxin A and an S1(m) gene sequence to a baculovirus vector to obtain a recombinant vector; carrying out recombinant vector site specificity transposition on a seroconversion DH10Bac competent cell of the recombinant vector to obtain a recombinant bacmid; transfecting the recombinant bacmid to an Sf9 cell under the mediation of a liposome to obtain a recombinant Sf9 cell; expressing by using the recombinant Sf9 cell. According to the invention, a PE(delta III)-S1(m)-KDEL3 fusion protein is firstly expressed by using a baculovirus expression system on the basis of optimizing the predilection of a codon of mammalian with S1 genes, so that the recombinant baculovirus for efficiently and accurately expressing the fusion protein is obtained. Proved by IFA and Western blot, the recombinant baculovirus can be used for accurately expressing the fusion protein, and the fusion protein has favorable reactionogenicity.
Owner:NANJING AGRICULTURAL UNIVERSITY
Compositions and methods for simultaneous detection of hcv antigen/antibody
Compositions and processes are provided for the robust detection of hepatitis C virus in a sample. Compositions include amino acid sequences of HCV core region including or exclusive to residues 14-31 or 50-90 of the HCV core protein. Also provided are processes of detecting HCV in a sample whereby the provided peptides are optionally used alone or in conjunction with antibodies that do not recognize the peptides so that detection is independent of seroconversion in a subject. Using the compositions and processes, HCV can be detected in a sample prior to or following seroconversion leading to robust HCV detection and diagnosis.
Owner:UNITED STATES OF AMERICA
Application of mimic antigen compound in treatment of HBV infection related symptoms
InactiveCN108283715ALower levelRepair damageViral antigen ingredientsDigestive systemAntigenSeroconversion
The invention relates to application of a mimic antigen compound in treatment of HBV infection related symptoms. The method is characterized in that mimic antigen compound with effective treatment dosage and containing the following two parts is applied to testers with the HBV infection related symptoms: (1) epsilon-CH3(CH2)14CO-(NH)-KSSQYIKANSKFIGITE and (2) AAAFLPSDFFPSVGGGDPRVRGLYFPA. With theadoption of the method, serum HBeAb seroconversion of a HBV infection patient and HBeAg / HBeAb serological transformation are induced, so that the serum HBV DNA level of the patient can be reduced, the serum ALT level is reduced, and the damaged liver cells can be recovered. Therefore, the method can be treated as an effective method for treating the HBV infection related symptoms.
Owner:ARMY MEDICAL UNIV
Compositions and methods for simultaneous detection of HCV antigen/antibody
Owner:UNITED STATES OF AMERICA
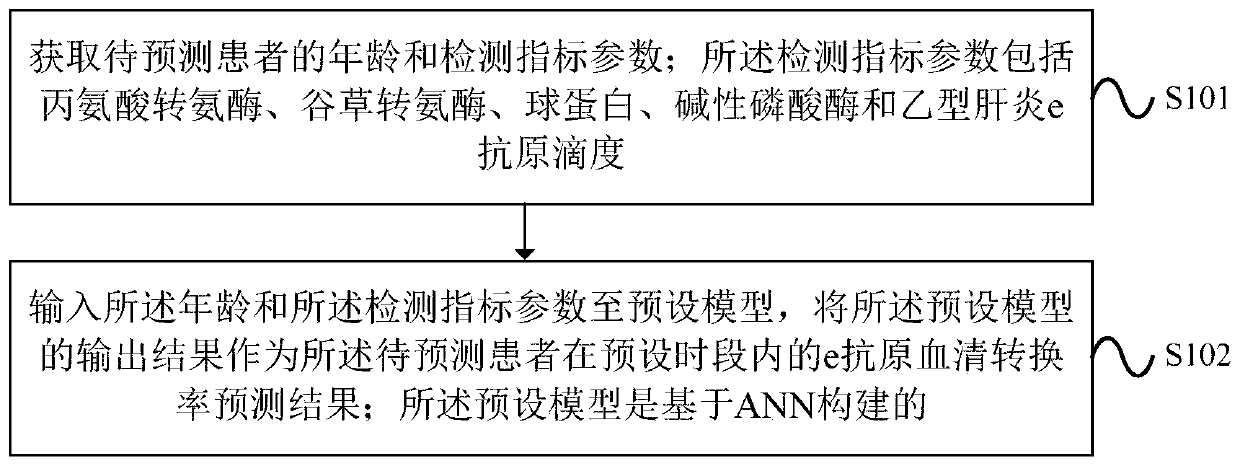
Processing method for predicting e-antigen seroconversion rate and device thereof
PendingCN109767839AImprove accuracyMedical simulationNeural architecturesHepatitis b e antigenSeroconversion
An embodiment of the invention provides a processing method for predicting an e-antigen seroconversion rate and a device thereof. The method comprises the steps of acquiring an age and detecting indexparameters of a to-be-predicted patient; wherein the detecting index parameters comprise alanine aminotransferase, glutamic oxaloacetic transaminase, globulin, alkaline phosphatase and hepatitis b e-antigen titer; inputting the age and the detecting index parameter into a preset model, using the output result of the preset model as an e-antigen seroconversion rate predicting result of the to-be-predicted patient in a preset time period; wherein the preset model is constructed based on ANN. The device executes the method. According to the processing method for predicting the e-antigen seroconversion rate and the device, through using the output result of the preset model which is constructed based on ANN as an e-antigen seroconversion rate predicting result of the to-be-predicted patient in the preset time period, accuracy in predicting the e-antigen seroconversion rate can be improved.
Owner:BEIJING DITAN HOSPITAL CAPITAL MEDICAL UNIV
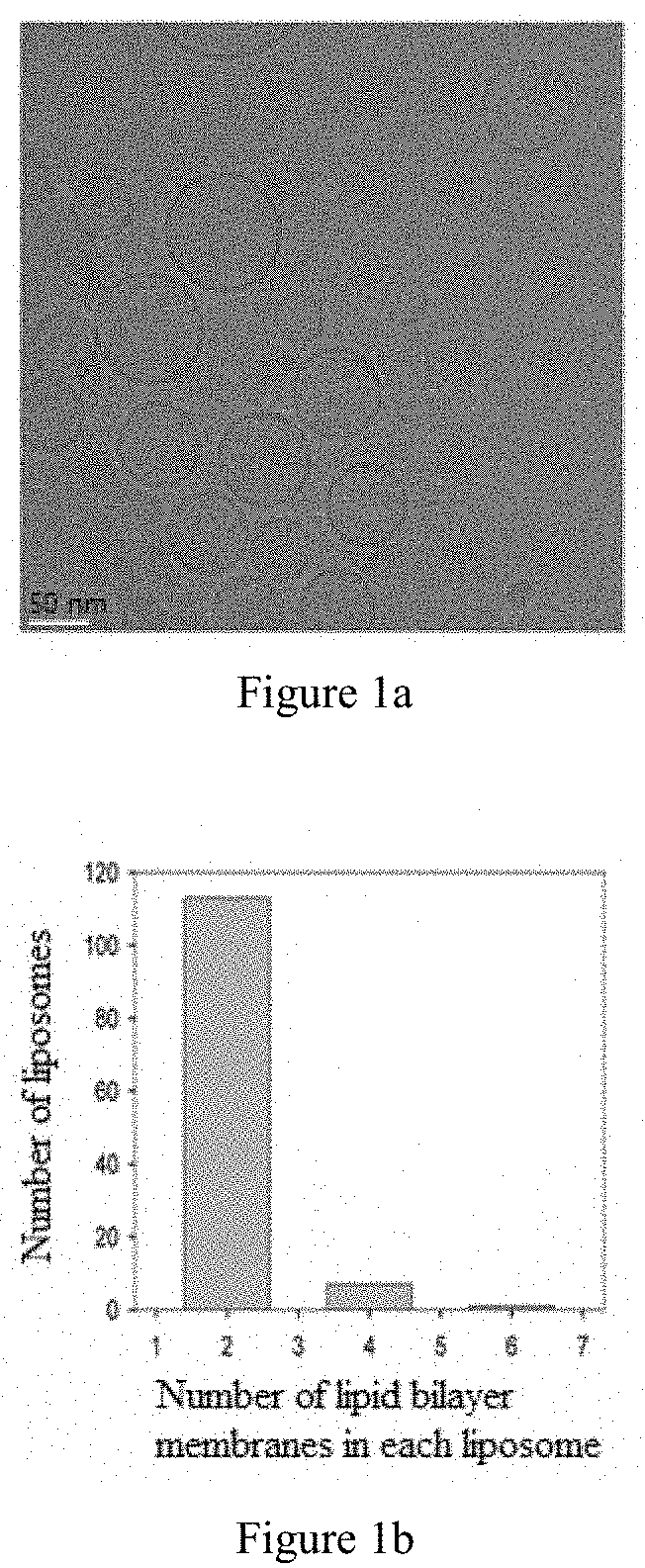
Use of liposomes for treatment of chronic viral hepatitis b
InactiveUS20200101019A1Simple and convenient and easy to operate processImprove stabilityPowder deliveryOrganic active ingredientsAntigenAntiendomysial antibodies
The invention relates to medicinal use of liposomes for the treatment of chronic viral hepatitis B, wherein said liposomes are prepared from phospholipid and cholesterol. This invention further relates to uses of said liposomes for the manufacture of medicaments for promoting the seroconversion of Hepatitis B e antibody to positive and the seroconversion of Hepatitis B e antigen to negative in patients with chronic viral hepatitis B, for reducing the hepatitis B virus titer in the peripheral blood serum of patients with chronic viral hepatitis B, for reducing the concentration of glutamic-pyruvic transaminase in the peripheral blood serum of patients with chronic viral hepatitis B, and for maintaining the stability of the T cell receptor repertoire in patients with chronic viral hepatitis B.
Owner:JIANGSU MENDEL GENETIC TECH INC +1
Methods for diagnosing human immunodeficiency virus infections
Provided is a method for identifying human immunodeficiency virus (HIV) in a sample. The invention involves use of a plurality of fluorescently labeled oligonucleotide probes and flow cytometry to detect the presence or absence of HIV in macrophages that are obtained from a mucosal surface. The invention is particularly useful for detecting HIV infection prior to seroconversion.
Owner:CORNELL UNIVERSITY +1
Vaccine against mumps containing a JERYL-LYNN virus strain
InactiveCN101092609AProtective responseCompensate for interferenceAntiviralsViruses/bacteriophagesSerum igeJeryl Lynn
A new mumps vaccine is presented, comprising a homogeneous pure isolate derived from the Jeryl-Lynn strain of mumps virus. In a preferred embodiment of the invention the vaccine produces higher seroconversion and antibody titres than known commercial vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Intradermal influenza vaccine
InactiveUS20130183340A1Excellent immune organEffective absorptionSsRNA viruses negative-senseViral antigen ingredientsHemagglutininSeroconversion
The invention relates to virosome-based influenza vaccines for the manufacture of medicaments that are administered intradermally in humans. The invention provides (trivalent) compositions comprising low doses of hemagglutinin (HA) antigen in a virosomal preparation that fulfill the immune response standards with respect to seroconversion rates, GMT-fold increase and protection rates, for use in vaccination set-ups.
Owner:CRUCELL SWITZERLAND AG
Hepatitis B virus resistant traditional Chinese medicine composition and preparation method thereof
InactiveCN102178789AStrong reduction of HBV DNAPromote the productionDigestive systemAntiviralsSeroconversionToxic material
The invention relates to the field of traditional Chinese medicine, in particular to a hepatitis B virus resistant traditional Chinese medicine composition composed of the following concrete components in parts: 15-20 parts of radix astragali, 12-15 parts of bupleurum, 12-15 parts of phellodendron bark, 6-12 parts of picrorhiza rhizome, 15-20 parts of giant knotweed, 15-20 parts of dyers woad leaf, 15-20 parts of asket fern, 12-15 parts of white atractylodes rhizome, 15-20 parts of tuckahoe, 15-20 parts of oldenlandia diffusa, 15-20 parts of red sage root and 5-8 parts of liquorice. The hepatitis B virus resistant traditional Chinese medicine composition can be prepared into capsules, decoction or extract, has the effects of clearing away heat and toxic material, strengthening the spleen and removing dampness through diuresis, has very strong efficacies of resisting hepatitis B virus, reducing HBVDNA (Hepatitis B Virus Deoxyribose Nucleic Acid) and promoting seroconversion of HBsAg (Hepatitis B Surface Antigen), and has multiple effects of protecting the liver, preventing pathological changes and the like.
Owner:刘明述
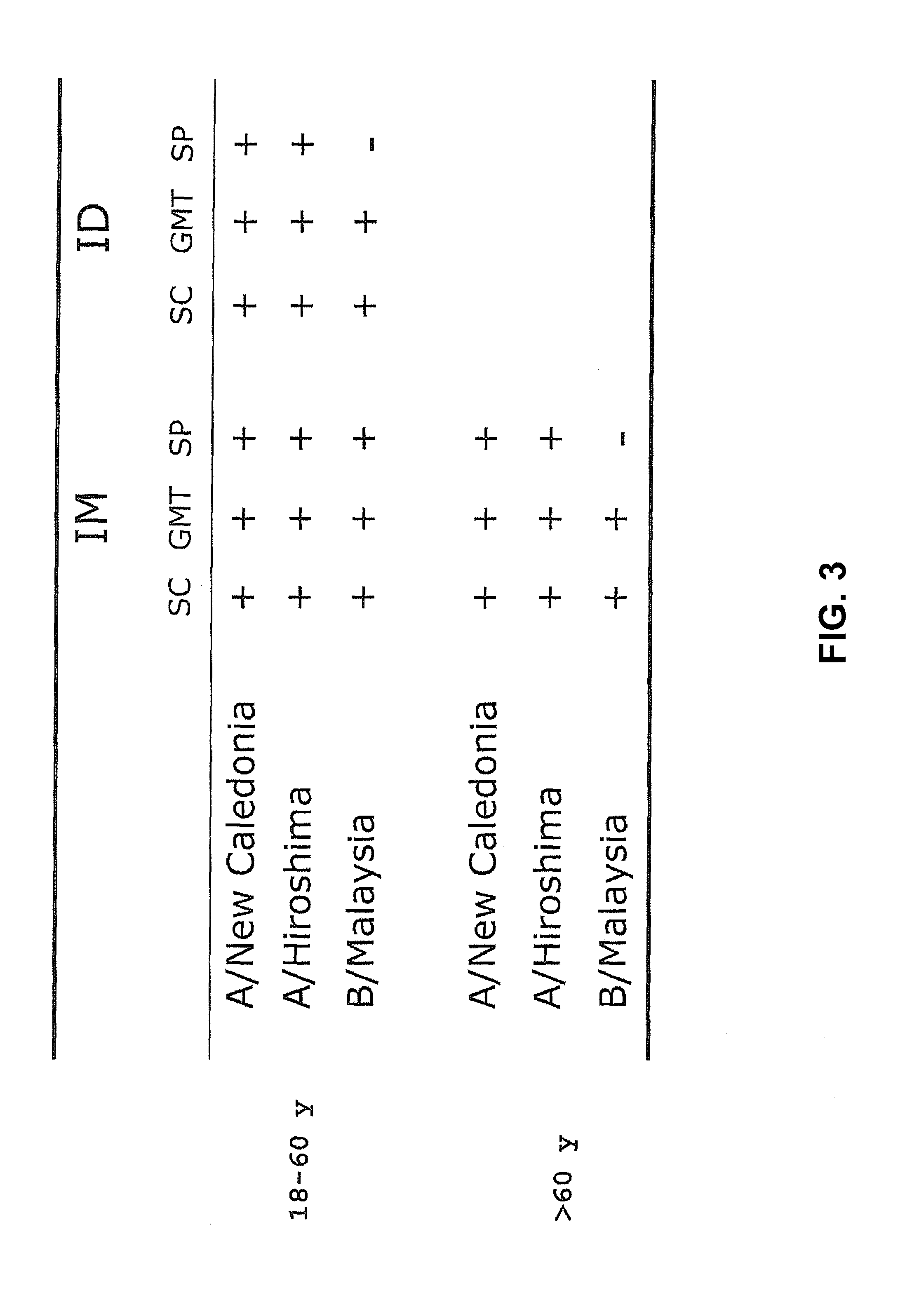
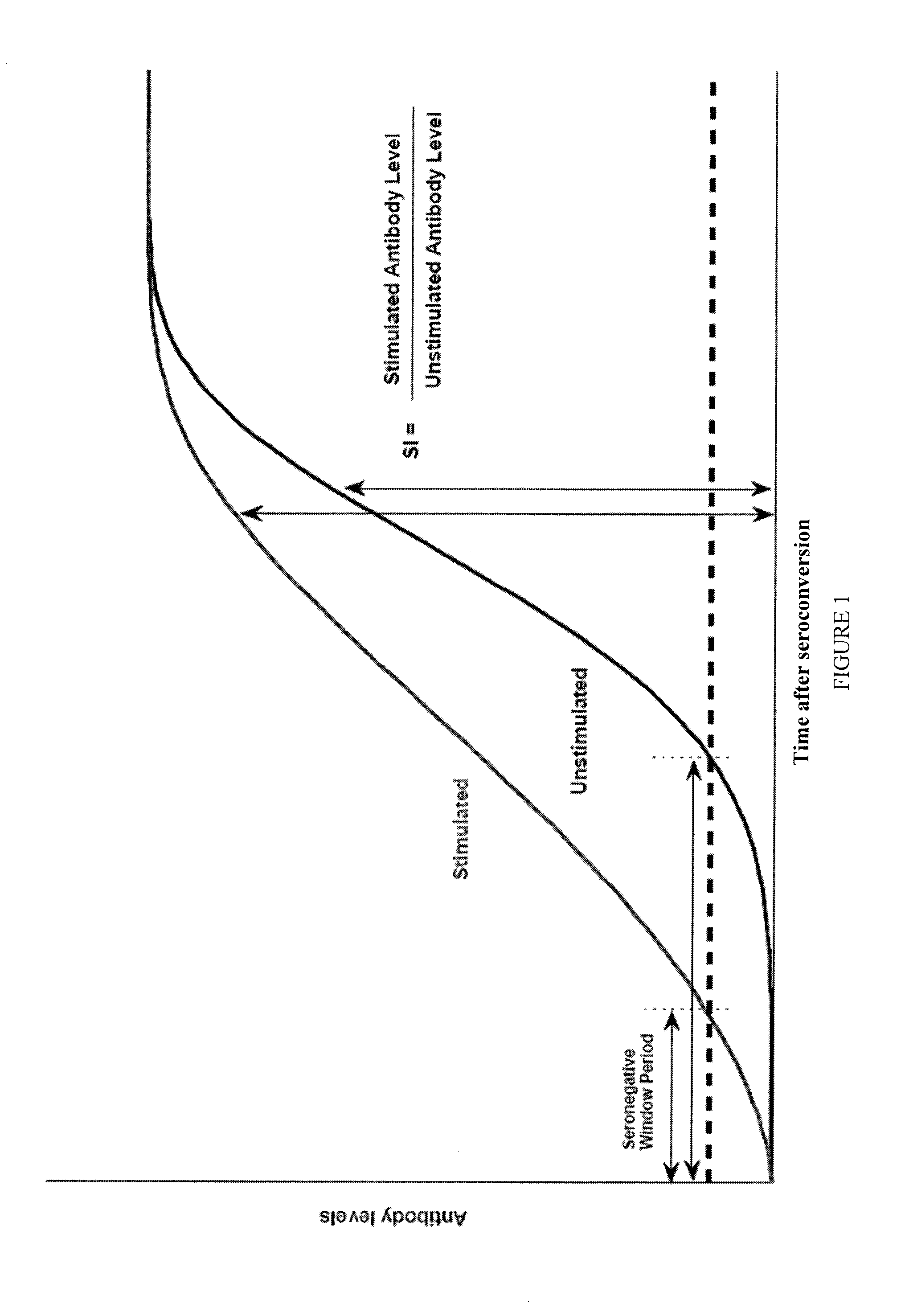
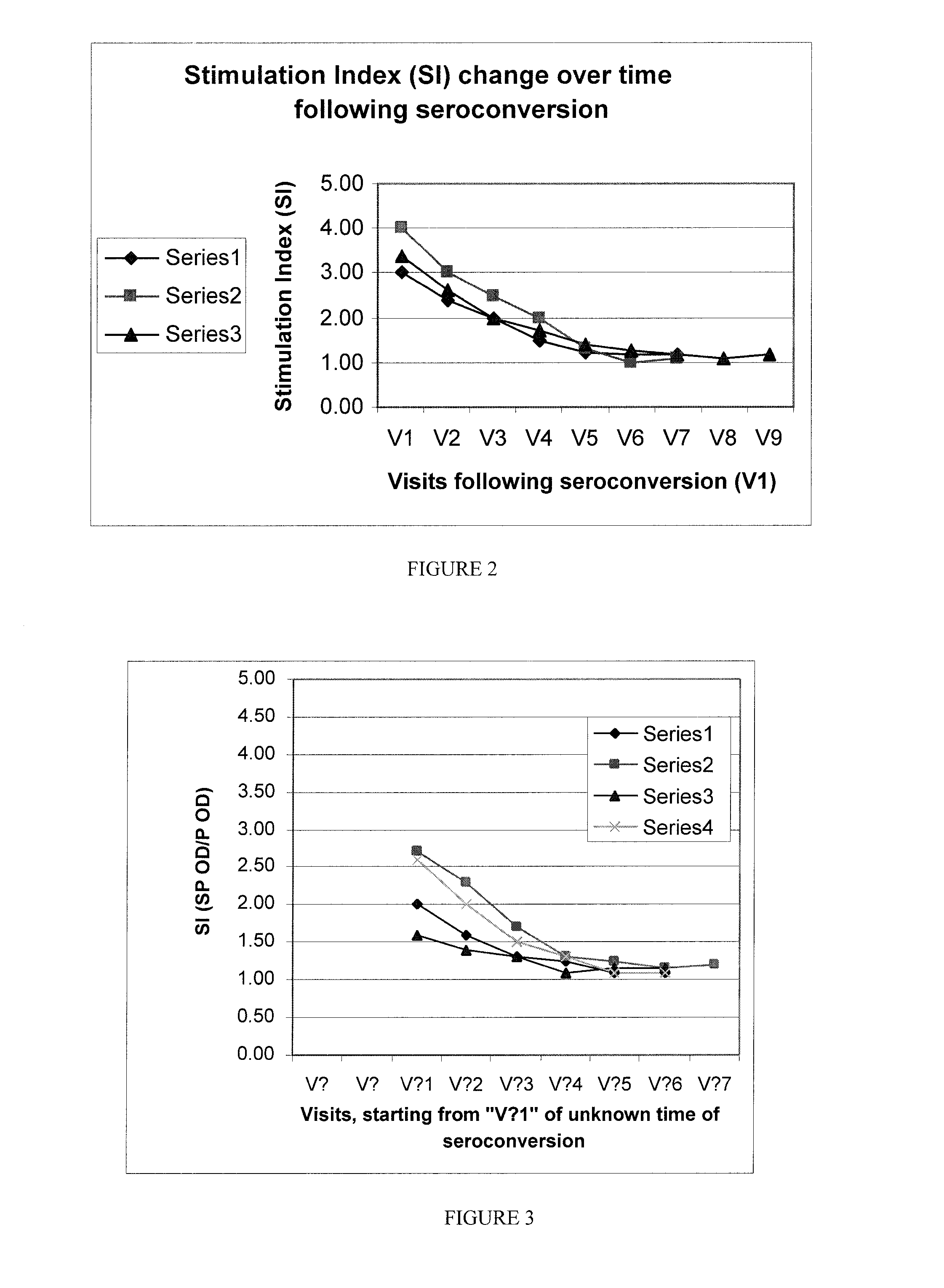
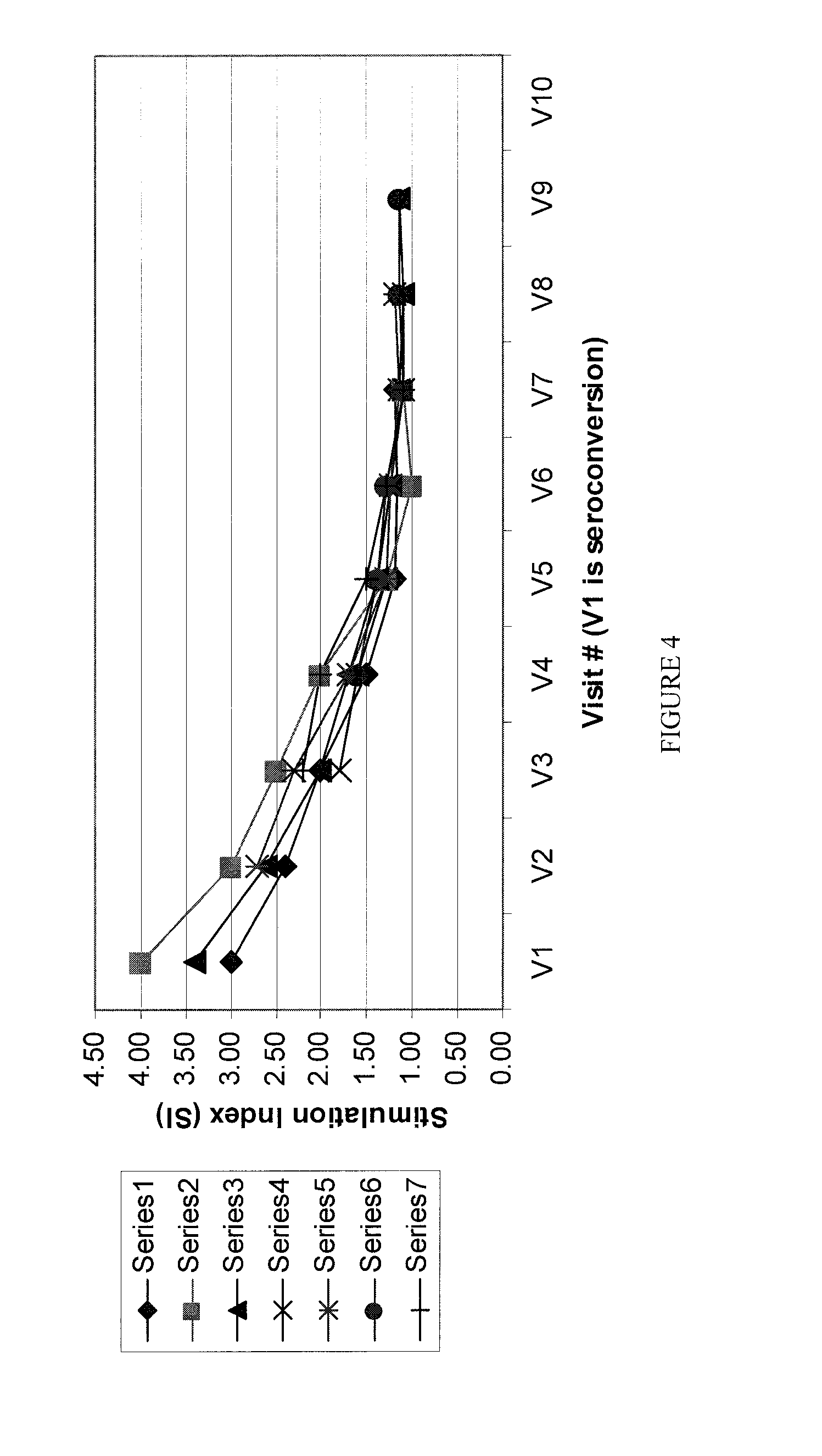
Method and kit for determining the time of seroconversion of a patient infected with a virus
The present invention provides a method for determining the time of infection, and a method for determining if a microbial infection is in the early stages comprising the step of determining the ratio of in vitro stimulated anti-microbial immunoreactivity and un-stimulated anti-microbial immunoreactivity in blood samples from said subject and related kits.
Owner:SMART BIOTECH
Vaccine against mumps containing a JERYL-LYNN virus strain
A new mumps vaccine is presented, comprising a homogeneous pure isolate derived from the Jeryl-Lynn strain of mumps virus. In a preferred embodiment of the invention the vaccine produces higher seroconversion and antibody titres than known commercial vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Methods of treating viral infection
ActiveUS11142795B2Peptide/protein ingredientsMicrobiological testing/measurementAntigenHepatitis B immunization
Disclosed herein is a novel use of C-type lectin 18 (CLEC18) in disease prognosis. According to embodiments of the present disclosure, the mRNA or protein level of CLEC18 may serve as an indicator for diagnosing hepatitis B virus (HBV) infection, hepatitis B e antigen (HBeAg) loss and seroconversion, and / or liver fibrosis.
Owner:ACAD SINIC
Method of Predicting Risk for Type 1 Diabetes
The present invention relates to methods, transcriptome profiles and kits useful for determining, before seroconversion, the risk that an individual will develop Type 1 diabetes (T1D).
Owner:TURUN YLIOPISTO
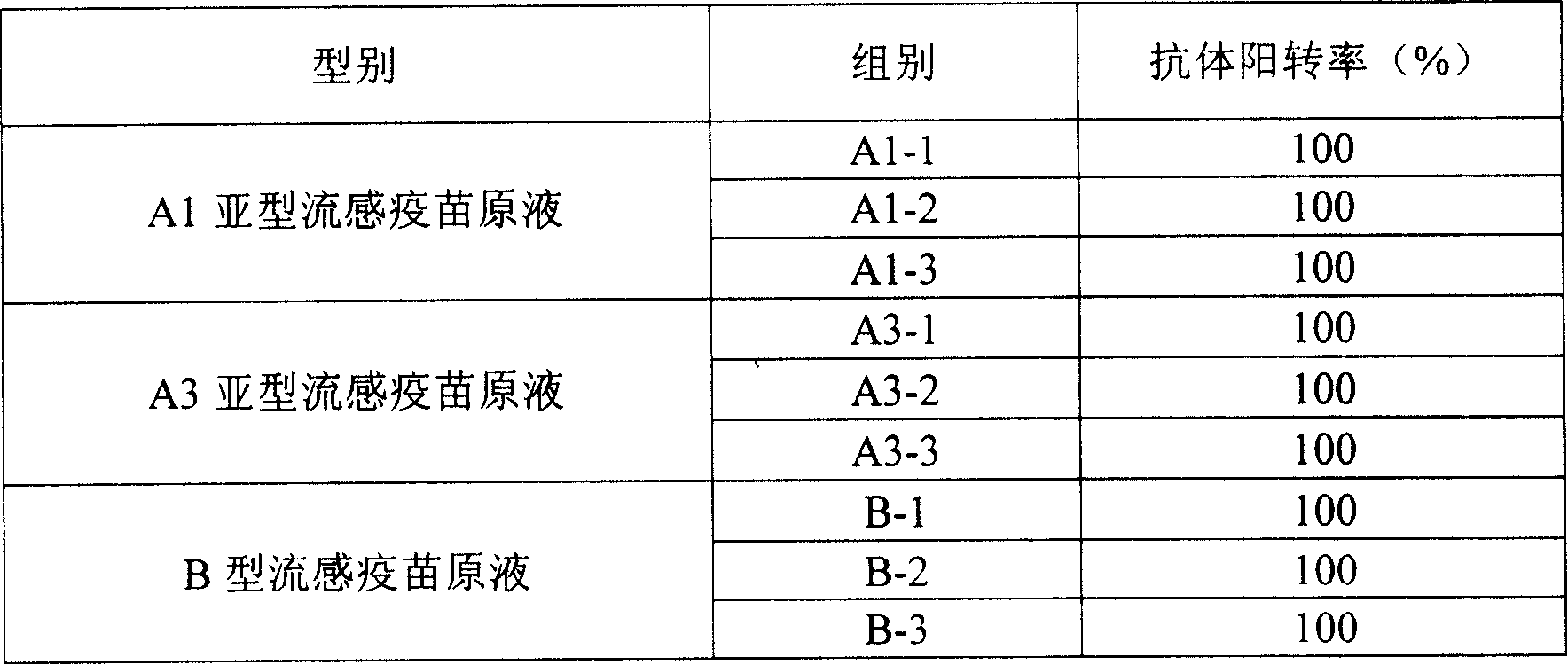
Method for preparing novel influenza vaccine
The invention relates to a process for preparing a novel influenza vaccine, belonging to the technical field of preparation technology of vaccine. The invention comprises that influenza viruses of A1 and A3 of hypotype and B-type are diluted via virus nutrient solution and are individually inoculated mammal cells which grows to single layer according to 1:10 <-1> -1:10 <-7>, yielding virus liquid which contains the influenza viruses. After being deactivated and purified, each virus primary liquid is obtained. The antigen content of each virus primary liquid is detected. The antigen contents of the virus primary liquids of influenza viruses of A1 and A3 of hypotype and B-type are respectively regulated to prepare the vaccine. The invention has the advantage that by employing the mammal cells to produce the influenza vaccines, the animal immunogenicity experiments of the product show that 15 micrograms of hemagglutinin is capable of enabling serums of all experimental animals to be in seroconversion, and the HI antibody titers are all over 1:40.
Owner:长春百克生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com