Application of mimic antigen compound in treatment of HBV infection related symptoms
A compound and antigen technology, applied in the direction of antibody medical components, virus antigen components, microorganisms, etc., to achieve the effect of reducing serum ALT levels and repairing damaged liver cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
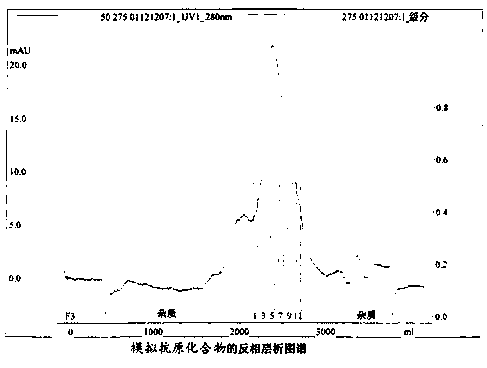
[0039] Example 1: Synthesis and purification of mimetic antigen compounds
[0040] 1) Synthesis of mimic antigen compounds
[0041] The sequence of the mimetic antigen compound of the present invention is ε-CH 3 (CH 2 ) 14 CO-(NH)-KSSQYIKANSKFIGITEAAFLPSDFFPSVGGGDPRVRGLYFPA was synthesized by Fmoc solid phase synthesis.
[0042] The fixed carboxy-terminus extends the peptide chain from the C-terminus to the N-terminus. Performed on an Applied Biosystems 431A peptide synthesizer. Fmoc amino acids are used as raw materials, the loading amount is 1mM, and the side chain protections are: Ser(tBu), Thr(tBu), Tyr(tBu), His(Trt), Gln(Trt), ASP(OtBu), Glu(OtBu), Arg(Pmc). HMP-resin was selected as the solid phase carrier, the loading amount was 0.25mM, and the raw material / resin ratio was 4:1. The amino acid coupled to the resin (the first amino acid at the carboxyl end of the polypeptide) is activated by the symmetrical anhydride activation method, and the activation of the re...
Embodiment 2
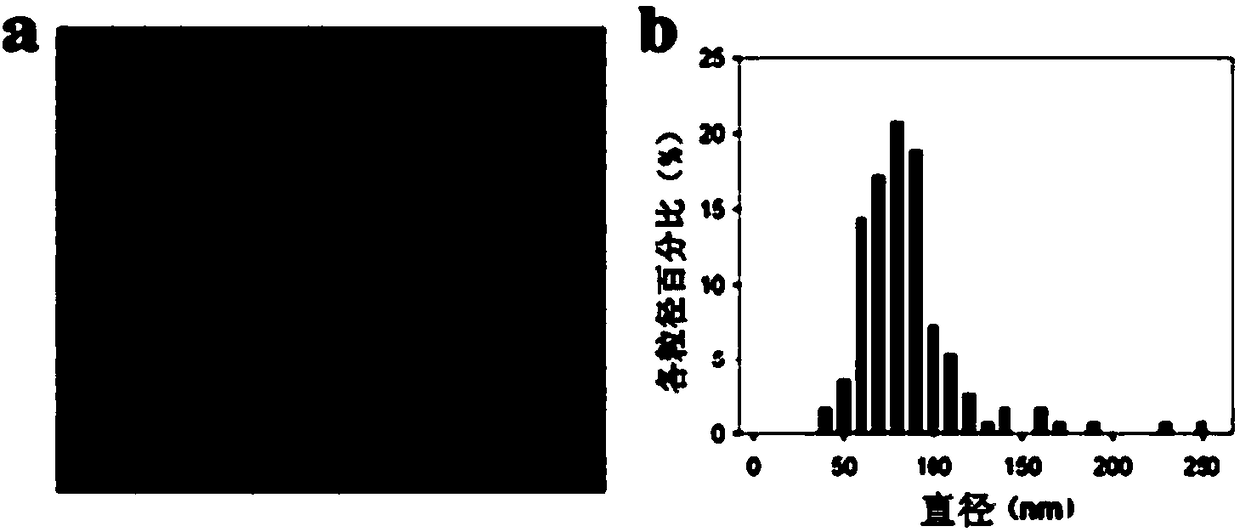
[0057] Embodiment 2: the preparation of mimetic antigen compound-liposome
[0058] 1) Preparation of mimetic antigen compound-liposome by secondary emulsification method: dissolve the lipid component in ether solution, then mix with the concentrated solution of mimetic antigen compound obtained in Example 1 to form an emulsion (W / O) , injected into phosphate buffered saline (PB) or water, while controlling the temperature and stirring, forming an emulsion (W / O / W) for the second time, and gradually forming the mimic antigen compound-liposome with the volatilization of ether. Through ultrafiltration device and dialysis (the dialysis ratio must reach more than 200 times) and 10μm microporous membrane filtration, to remove the mimetic antigen compound-liposome that may be free and possibly aggregated and precipitated in the liquid, that is, to obtain the mimetic antigen compound- Liposome Concentrate. Then add 140mL of 20% mannitol aqueous solution, 30mL of 20% human serum albumi...
Embodiment 3
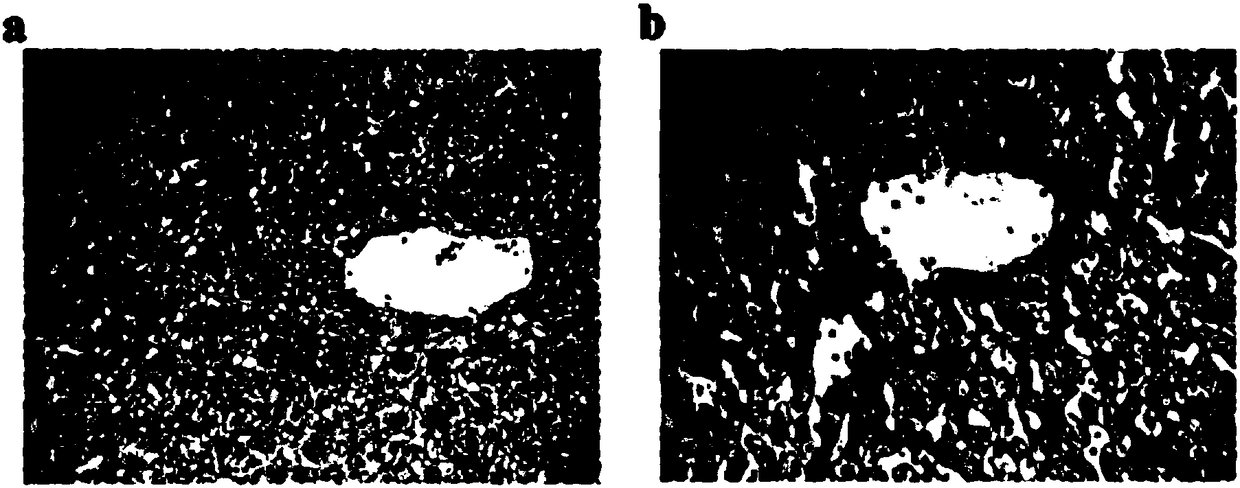
[0061] Embodiment 3: The therapeutic effect of mimetic antigen compound-liposome in chronic hepatitis B patient
[0062] In this example, the antigen-mimicking compound-liposome finished product prepared in Example 1 is used to treat selected patients with chronic hepatitis B, and to study its therapeutic effect and effect on chronic hepatitis B disease.
[0063] (1) Selection of subjects:
[0064] In the first stage (0-76 weeks), 360 HBeAg-positive chronic hepatitis B patients were selected as treatment objects. Divided into the following 3 groups, each group of 120 subjects: one group was given 600 μg mimic antigen compound-liposome+300 μg empty liposome (600 μg treatment group); one group was given 900 μg mimic antigen compound-liposome (900 μg treatment group). group); one group was given 900 μg of empty liposomes (control group).
[0065] The second stage (76-144 weeks): Among the three groups of subjects who completed the 76-week study above, if there is a virological ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com