Intradermal influenza vaccine
A technology of influenza vaccine and influenza, which is applied in the field of infectious diseases and drugs, and can solve the problems of non-use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
[0048] In another preferred embodiment, the vaccine comprises 1-10 μg of HA antigen from a single strain (or from each of the 3 influenza strains in the case of a trivalent vaccine), eg about 3.0 μg. In a more preferred embodiment, the virion-based influenza vaccine is Berna Biotech AG under the trademark V vaccine commercialized vaccine. Preferably, the invention relates to a method according to the invention, wherein the vaccine does not contain HA from influenza strains A / New Caledonia / 20 / 99, A / Moscow / 10 / 99 and B / Hong Kong / 330 / 2001 Trivalent combination of antigens. As indicated herein, administration is preferably performed using a delivery device suitable for intradermal delivery of the vaccine. A device suitable for intradermal delivery may be a single hypodermic needle. It was found that some devices were prepared so that the needle could not pass through the cortex, which would result in sub-optimal intradermal delivery. In other words, some devices contain relati...
Embodiment 2
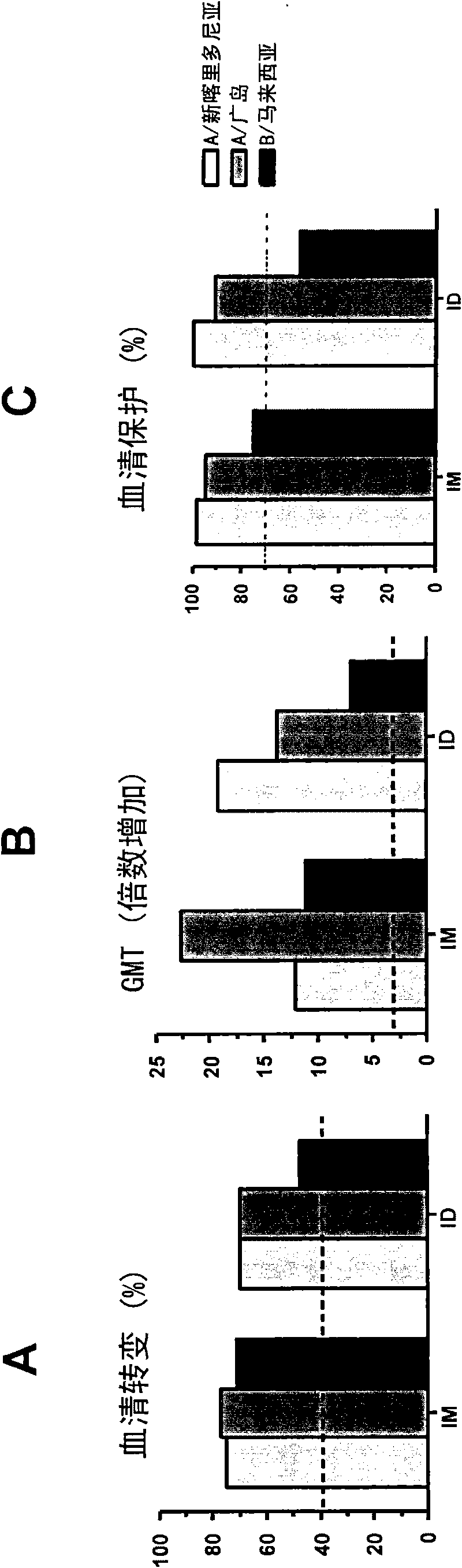
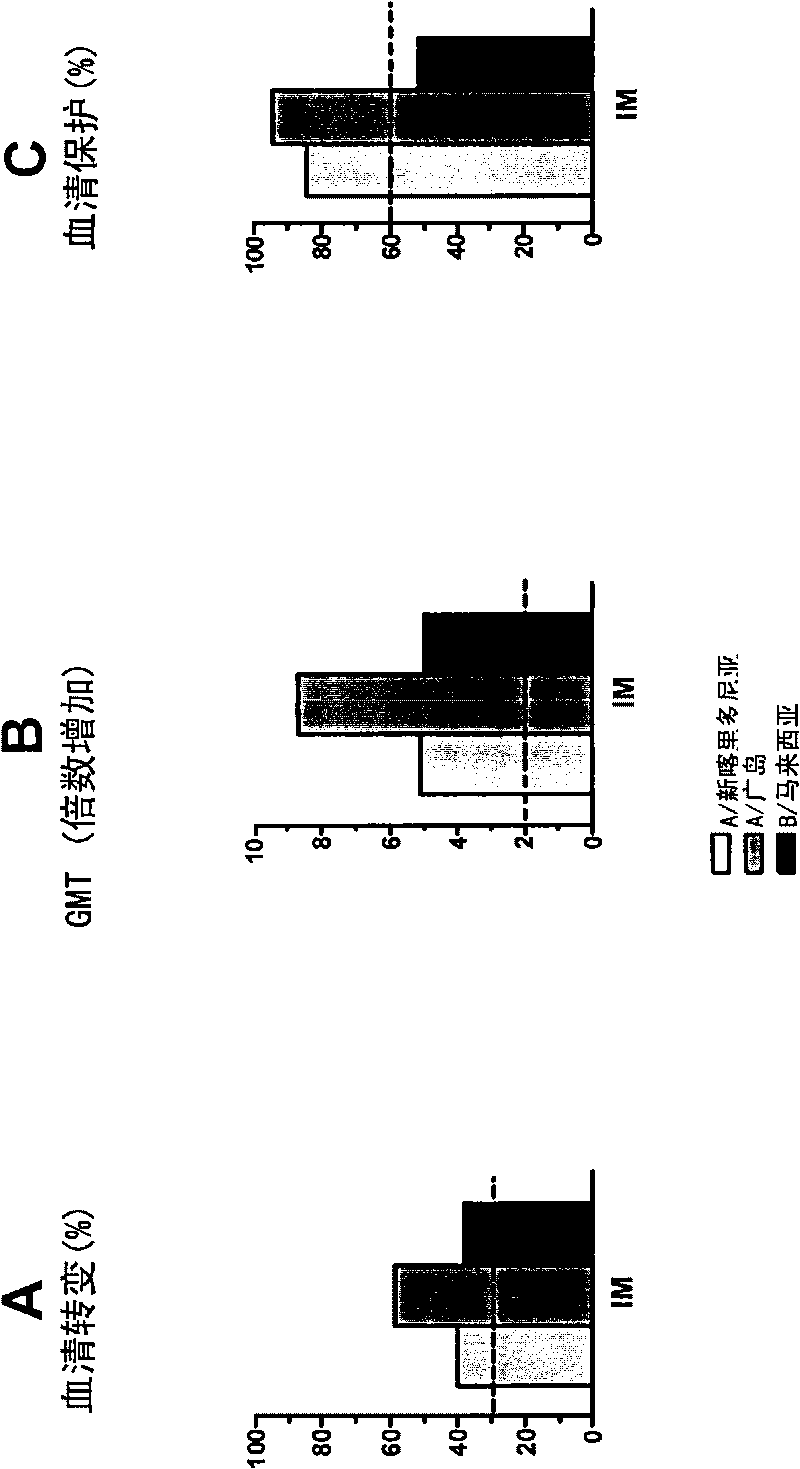
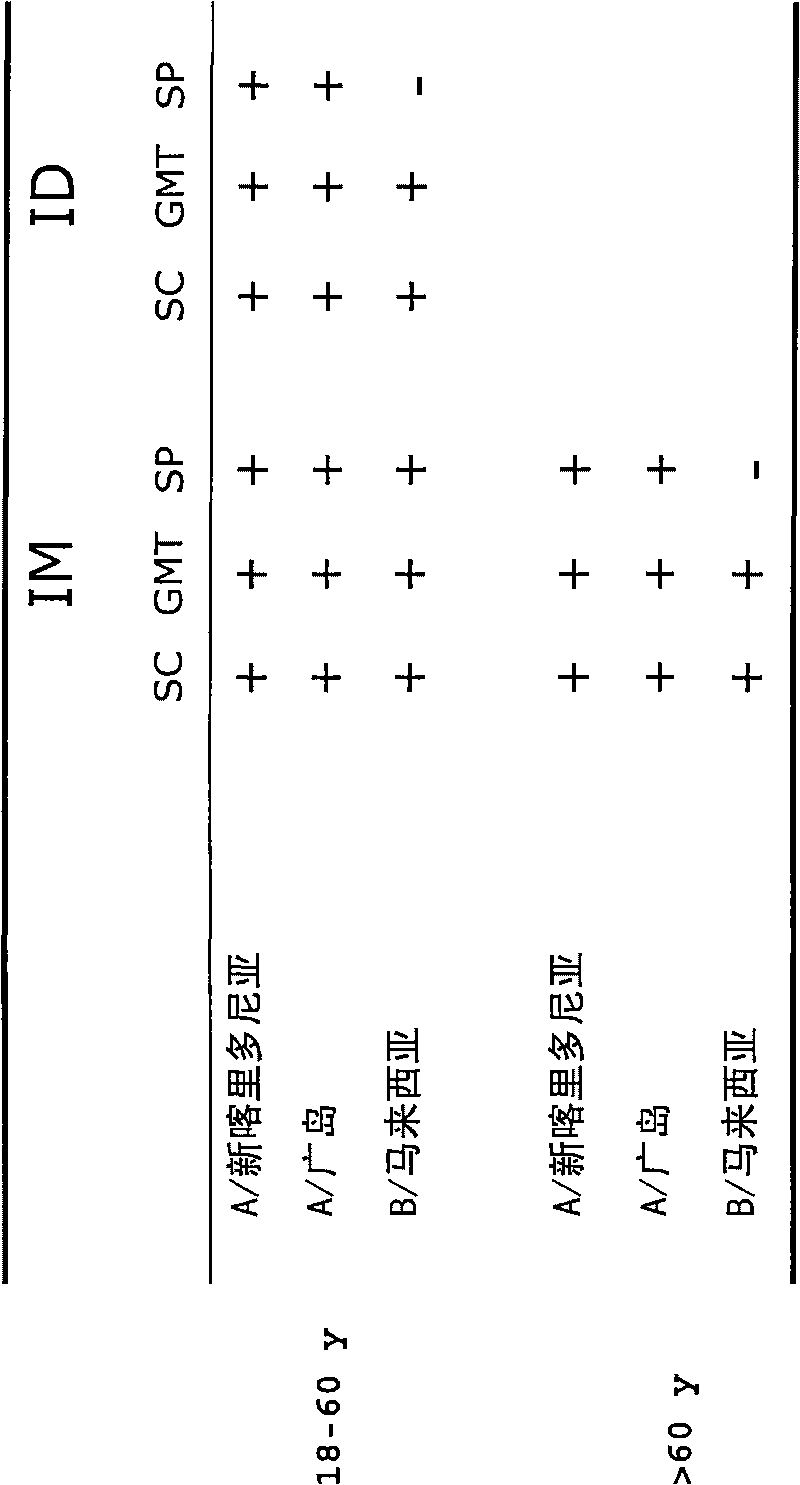
[0051] These studies showed that when virion-based influenza vaccines were administered intradermally to humans at a concentration of 3 μg of each strain (two A strains and one B strain), seroconversion and GMT criteria were positive for all three strains. is met, and the seroprotection rate of at least two A strains is met. This is very unexpected and in stark contrast to what was disclosed in WO2004 / 016281, where it was shown that this type of vaccination did not meet the standards set by the authorities for any of the three strains used therein. Example 2. Dose Escalation (and Intramuscular vs. Intradermal) Studies with Virosome-Based Influenza Vaccines in Human Individuals
[0052] A second clinical study involving human subjects was conducted to evaluate the humoral immune response to an intradermally administered seasonal virion-adjuvanted influenza vaccine. This included a single-center, randomized dose-escalation study in which a volume of 0.1 mL of trivalent V Infl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com