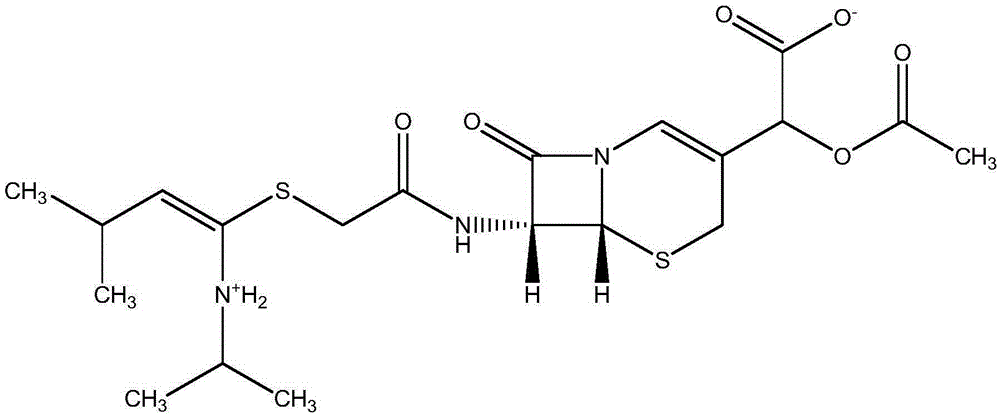
A novel industrial crystallization method of cefathiamidine and its preparation
A cefathiamidine and industrial crystallization technology, applied in the field of medicine, can solve the problems of unstable quality, many impurities and few impurities, and achieve the effects of high separation efficiency, high crystallization efficiency and short crystallization time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
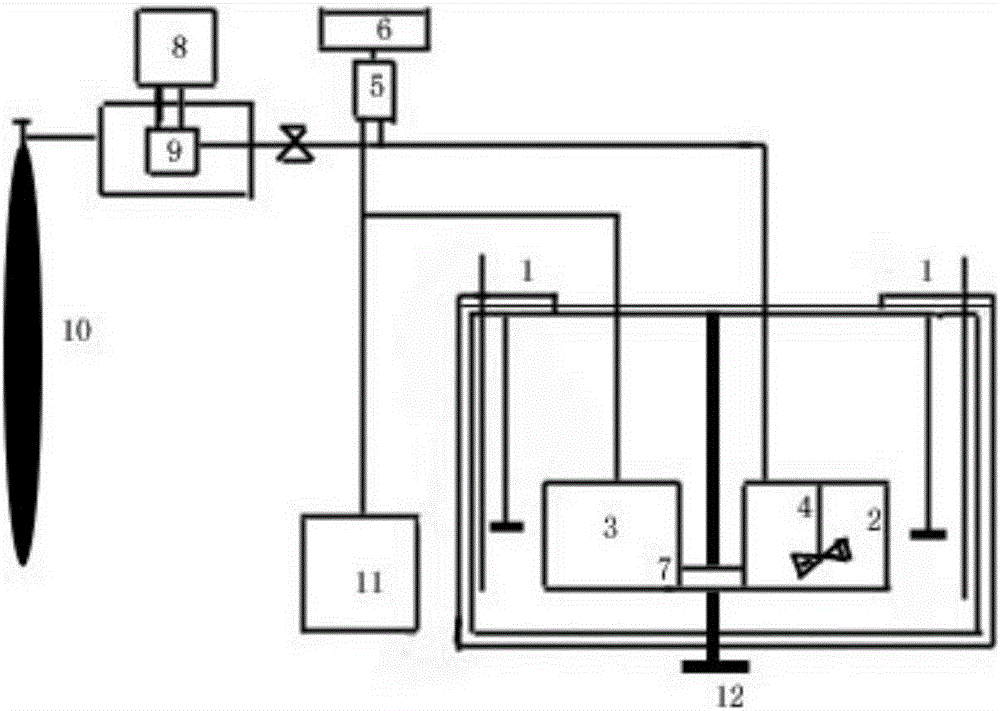
Image
Examples
Embodiment 1
[0044] (1) Weigh 6.79 kg of cefathiamidine crude product with a purity of 84.6% and place it in the extraction tank, add 60 kg of methanol-acetone mixed solvent with a volume ratio of 4:1, control the temperature at 50° C., and stir to dissolve it;
[0045] (2) Pump in the alkane fluid to 50Mpa with a high-pressure liquid pump, stir, and keep the pressure and temperature for 30 minutes, and turn off the high-pressure pump;
[0046] (3) Place crystal seeds in the crystallization pool, raise the height of the extraction pool to 30cm, open the quick interface of the two pools, let the liquid in the extraction pool enter the crystallization pool, and close the quick interface;
[0047] (4) Adjust the pressure in the crystallization tank to be 1Mpa, the temperature is 20°C, and keep this temperature and pressure for 40 minutes;
[0048] (5) After the system is cooled down and the pressure is released, dry under reduced pressure to obtain 5.16 kg of high-purity cefathiamidine crystall...
Embodiment 2
[0051] (1) Weigh 6.44 kg of cefathiamidine crude product with a purity of 84.6% and place it in the extraction tank, add 60 kg of methanol-acetone mixed solvent with a volume ratio of 1:2, control the temperature at 40°C, and stir to dissolve it;
[0052] (2) Pump in the alkane fluid to 30Mpa with a high-pressure liquid pump, stir, and keep the pressure and temperature for 10 minutes, and turn off the high-pressure pump;
[0053] (3) Place crystal seeds in the crystallization pool, raise the height of the extraction pool to 30cm, open the quick interface of the two pools, let the liquid in the extraction pool enter the crystallization pool, and close the quick interface;
[0054] (4) Adjust the pressure in the crystallization tank to 0.1Mpa, the temperature is 10°C, and keep the temperature and pressure for 20 minutes;
[0055] (5) After cooling down the system and releasing the pressure, dry under reduced pressure to obtain 4.87 kg of high-purity cefathiamidine crystalline pr...
Embodiment 3
[0058] (1) Weigh 5.66 kg of cefathiamidine crude product with a purity of 84.6% and place it in the extraction tank, add 60 kg of methanol-acetone mixed solvent with a volume ratio of 1:1, control the temperature at 40° C., and stir to dissolve it;
[0059] (2) Pump in the alkane fluid to 40Mpa with a high-pressure liquid pump, stir, and keep the pressure and temperature for 20 minutes, and turn off the high-pressure pump;
[0060] (3) Place crystal seeds in the crystallization pool, raise the height of the extraction pool to 20cm, open the quick interface of the two pools, make the liquid in the extraction pool enter the crystallization pool, and close the quick interface;
[0061] (4) Regulate the pressure in the crystallization tank to be 0.5Mpa, the temperature is 15°C, and keep this temperature and pressure for 30 minutes;
[0062] (5) After the system was cooled down and the pressure was relieved, it was dried under reduced pressure to obtain 4.13 kg of high-purity cefat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| stone rate | aaaaa | aaaaa |
| stone rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com