Patents
Literature
70 results about "Cefamandole nafate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
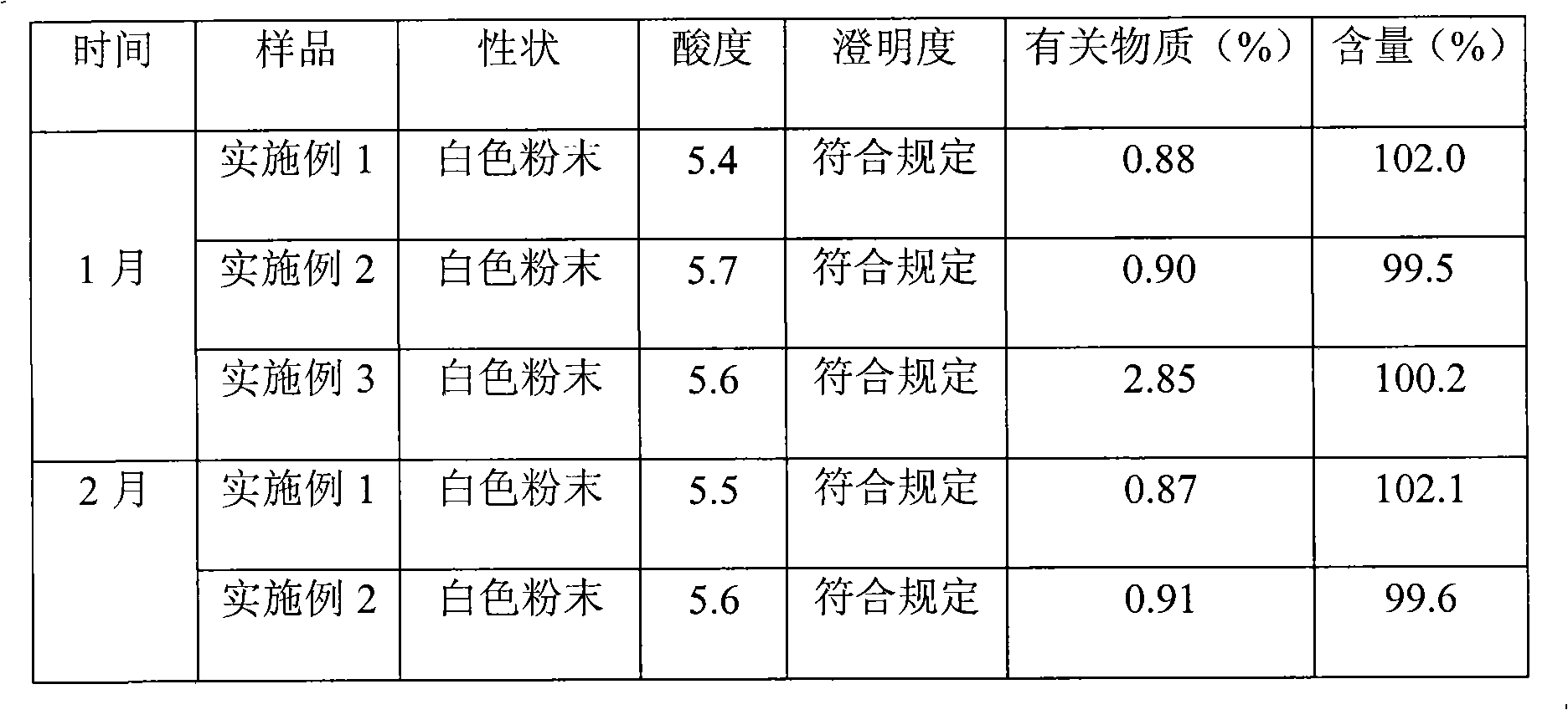
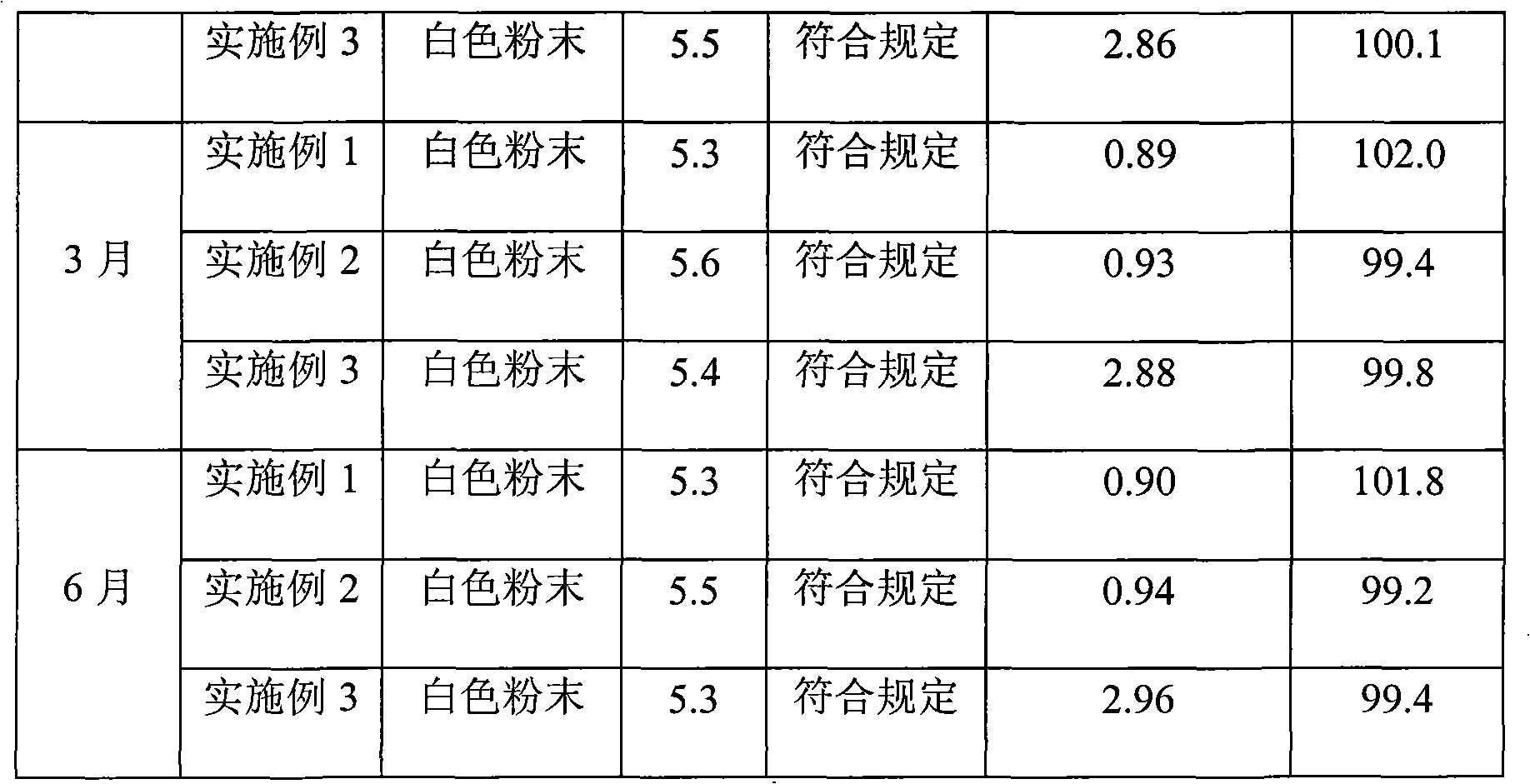
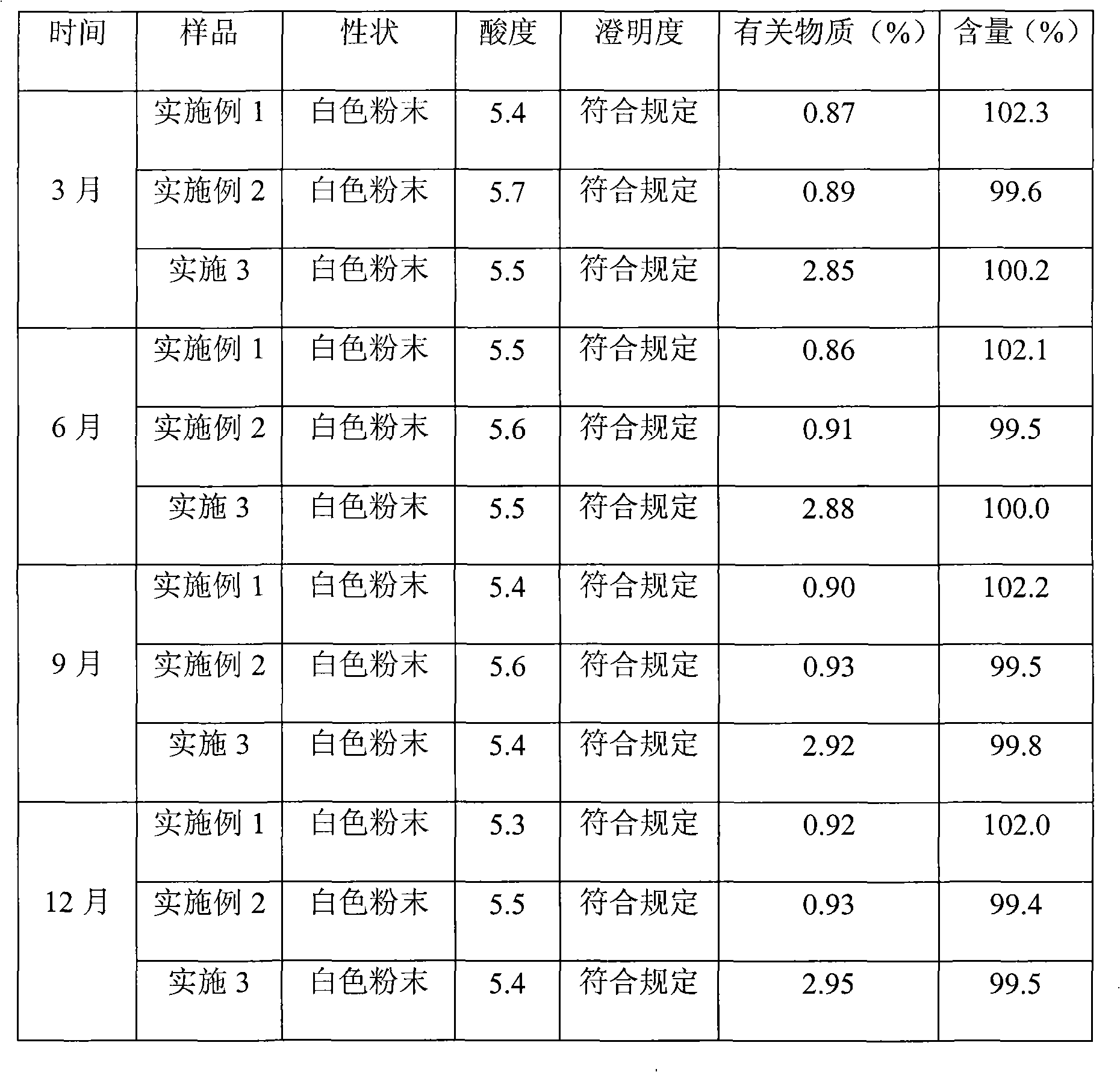
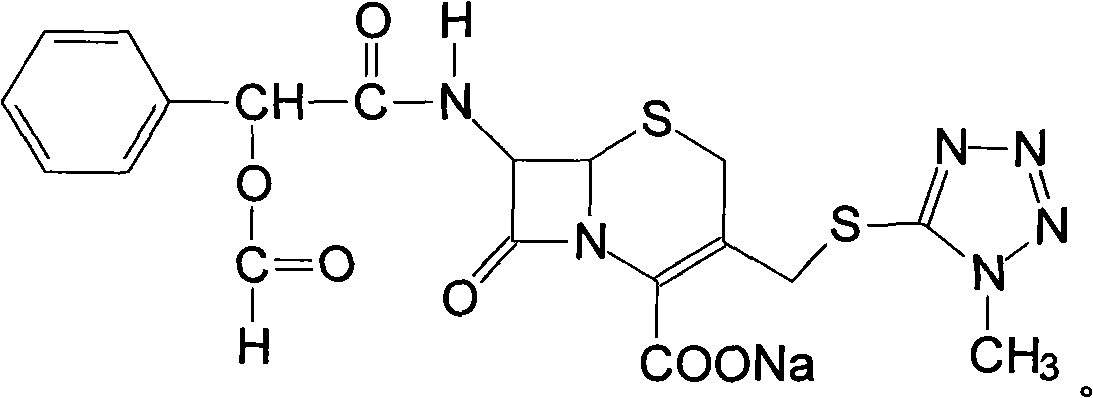
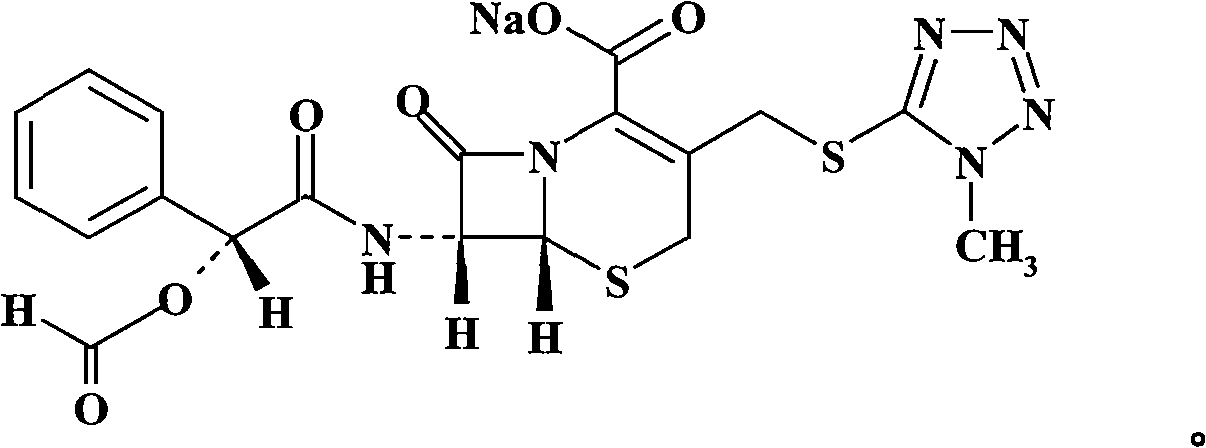
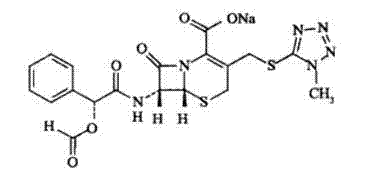
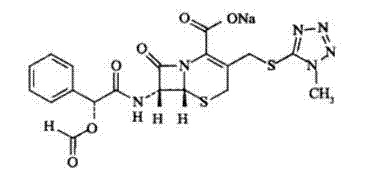
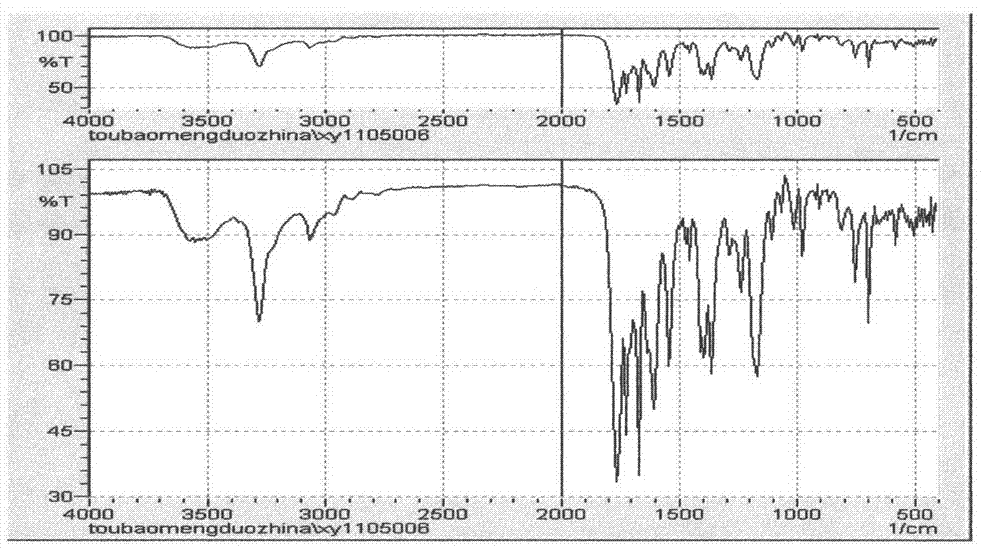
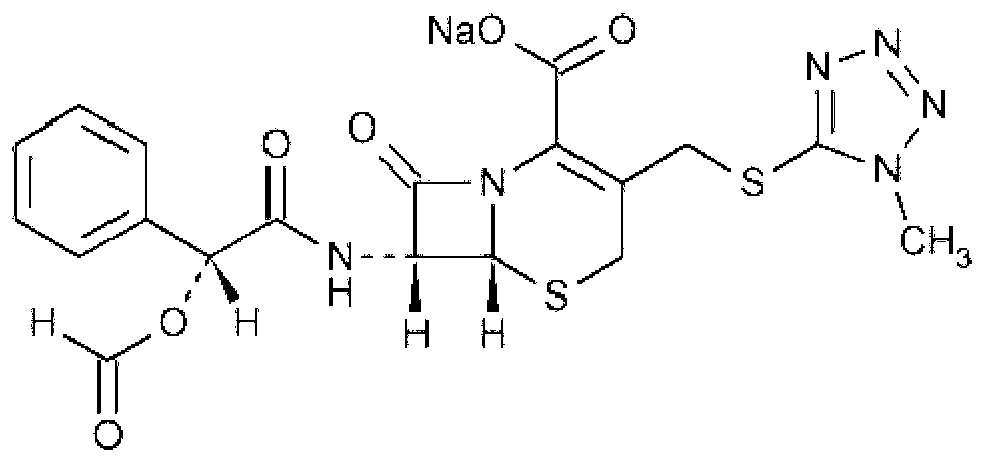
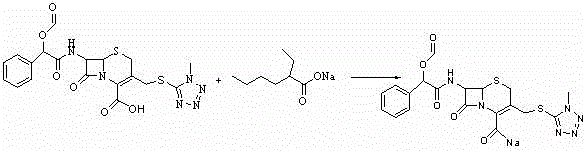
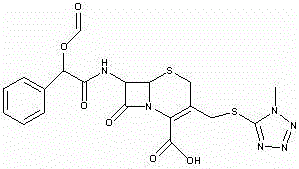
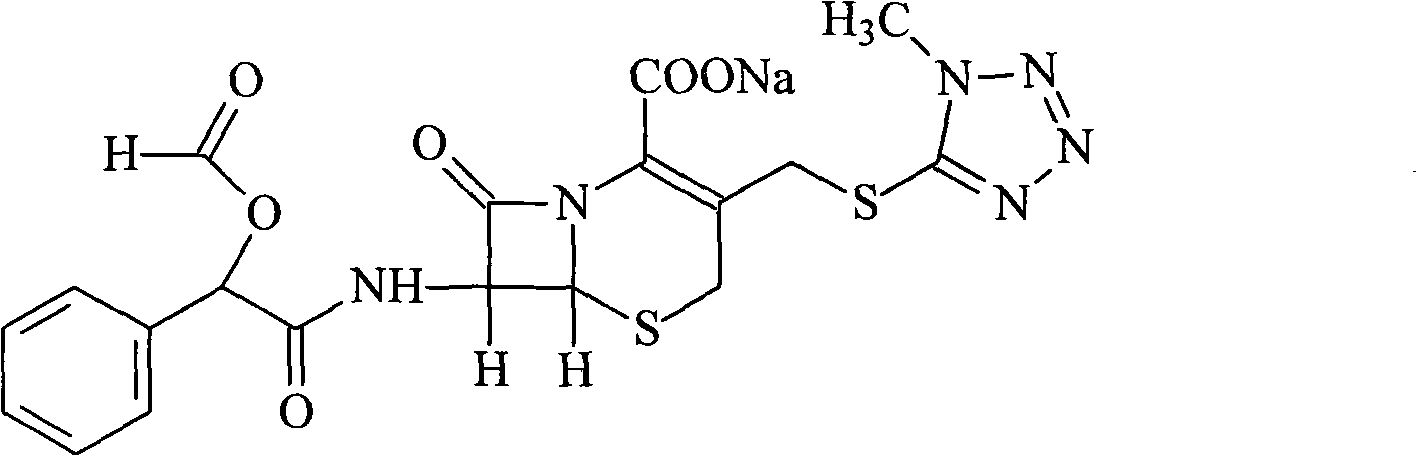
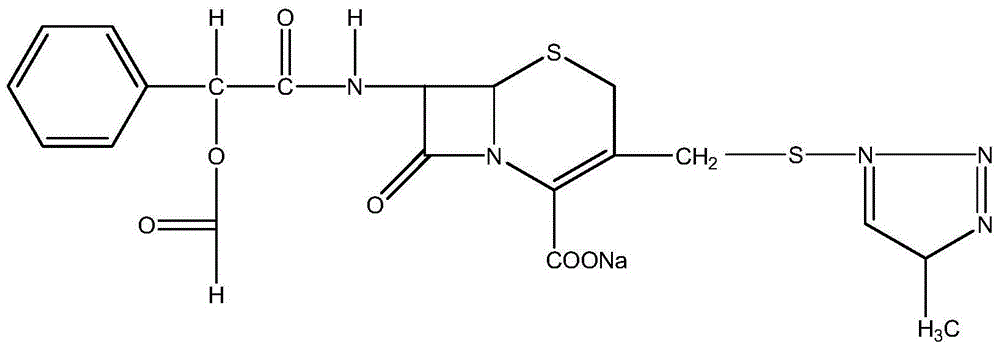
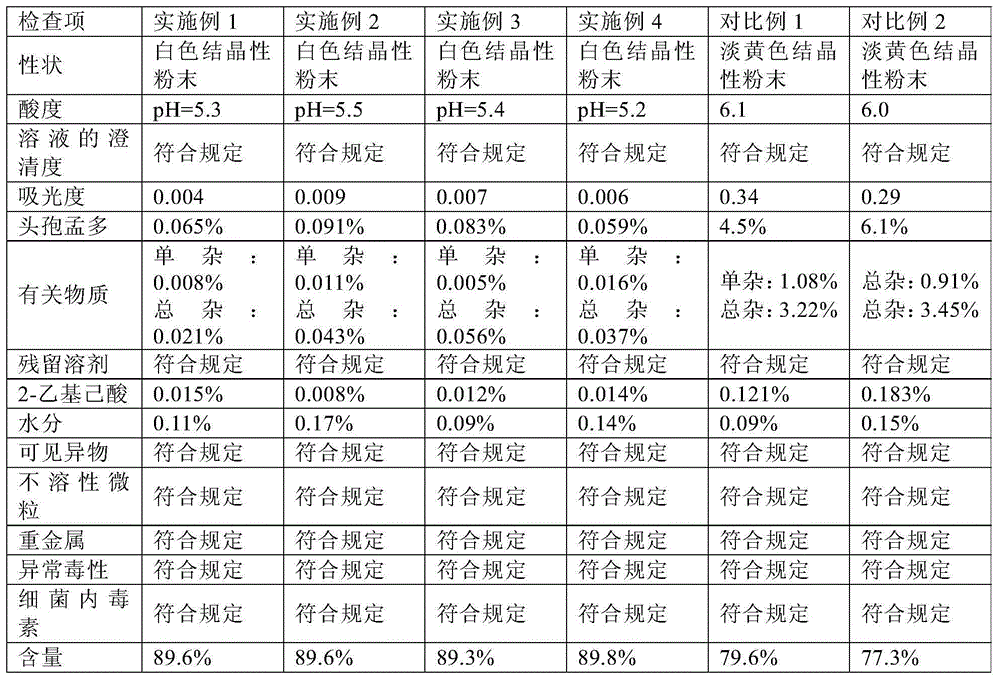
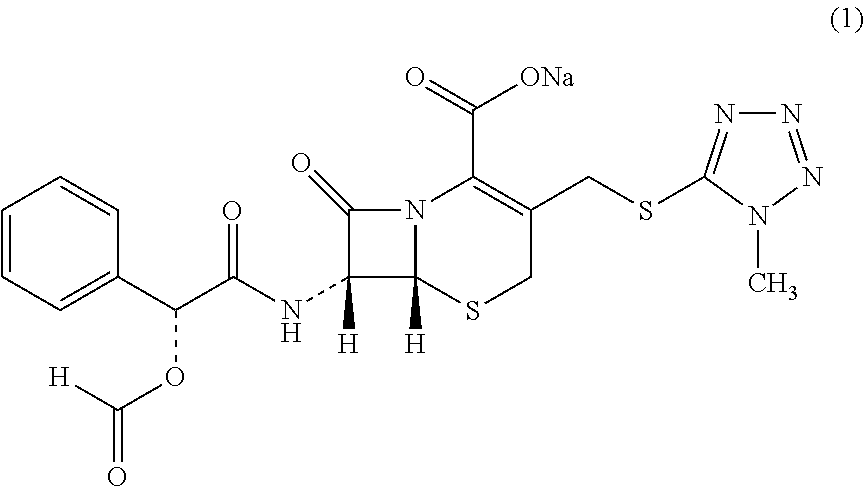
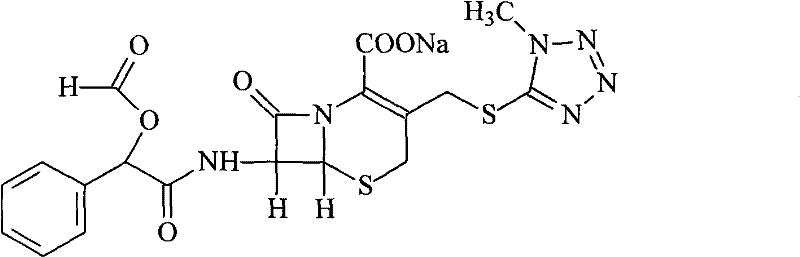
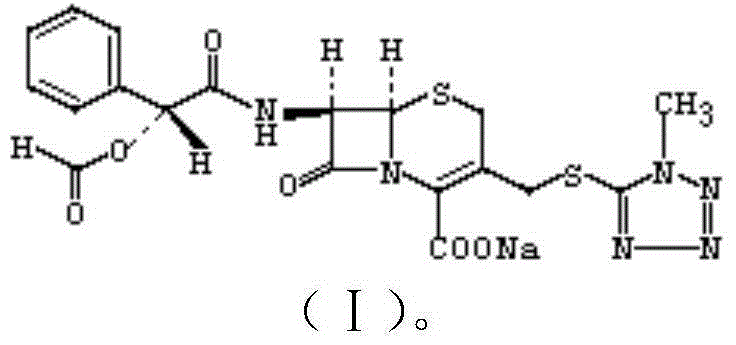
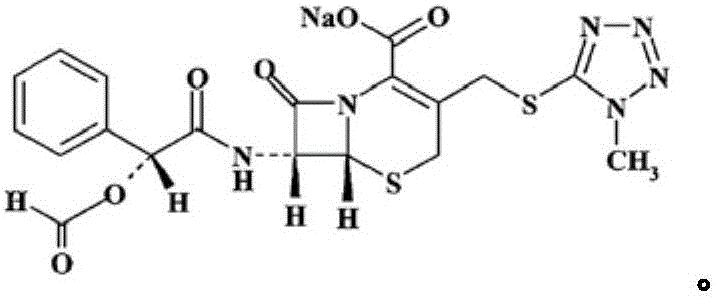
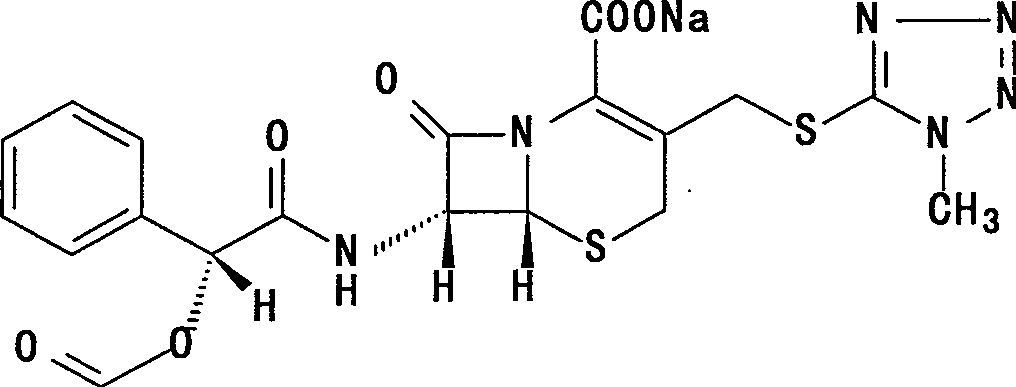
Cefamandole Nafate is the sodium salt form of cefamandole formyl ester. Cefamandole nafate is a pro-drug that is hydrolyzed by plasma esterases to produce cefamandole, a semi-synthetic beta-lactam, second-generation cephalosporin antibiotic with bactericidal activity.
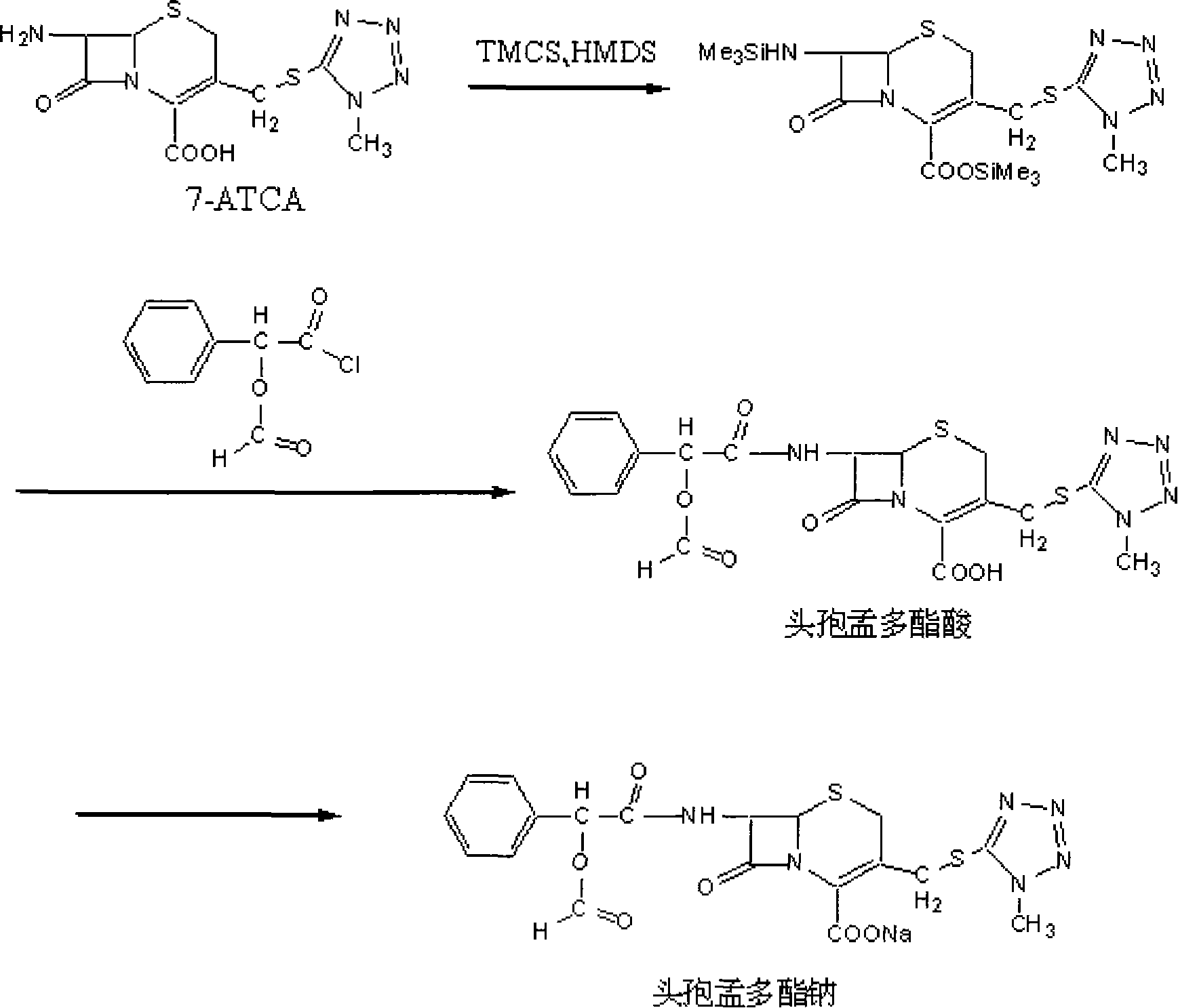
Method of synthesizing antibiotics cefamandole nafate
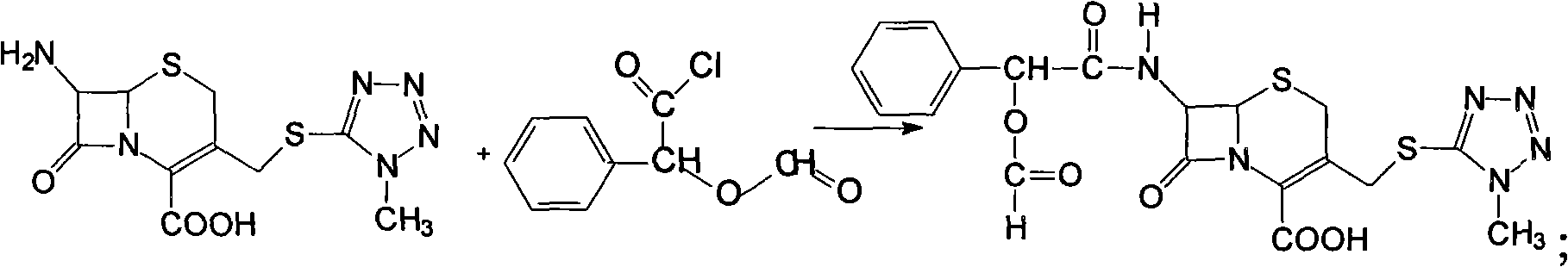
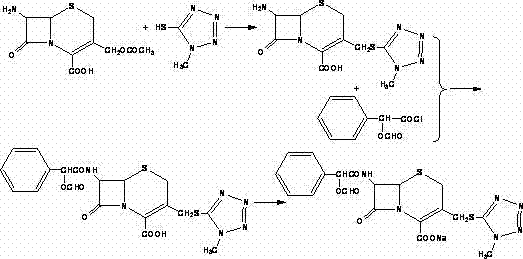
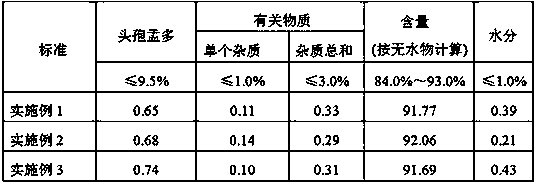
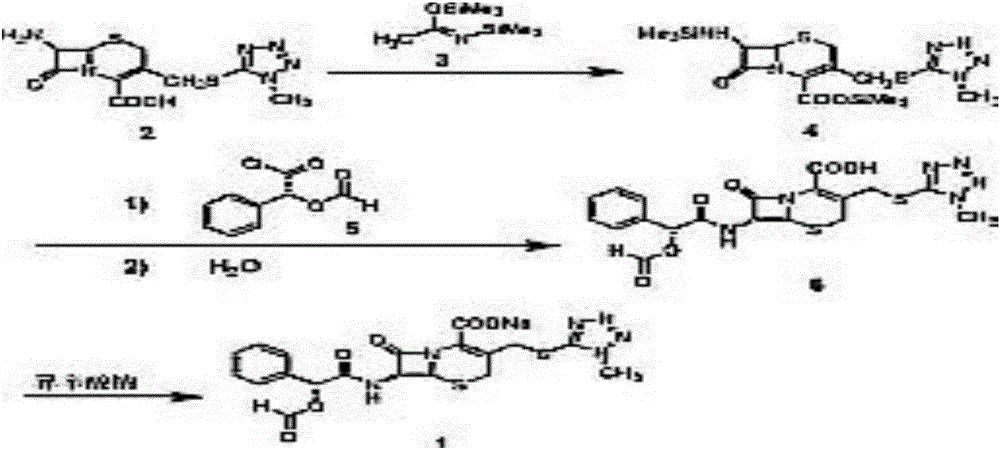
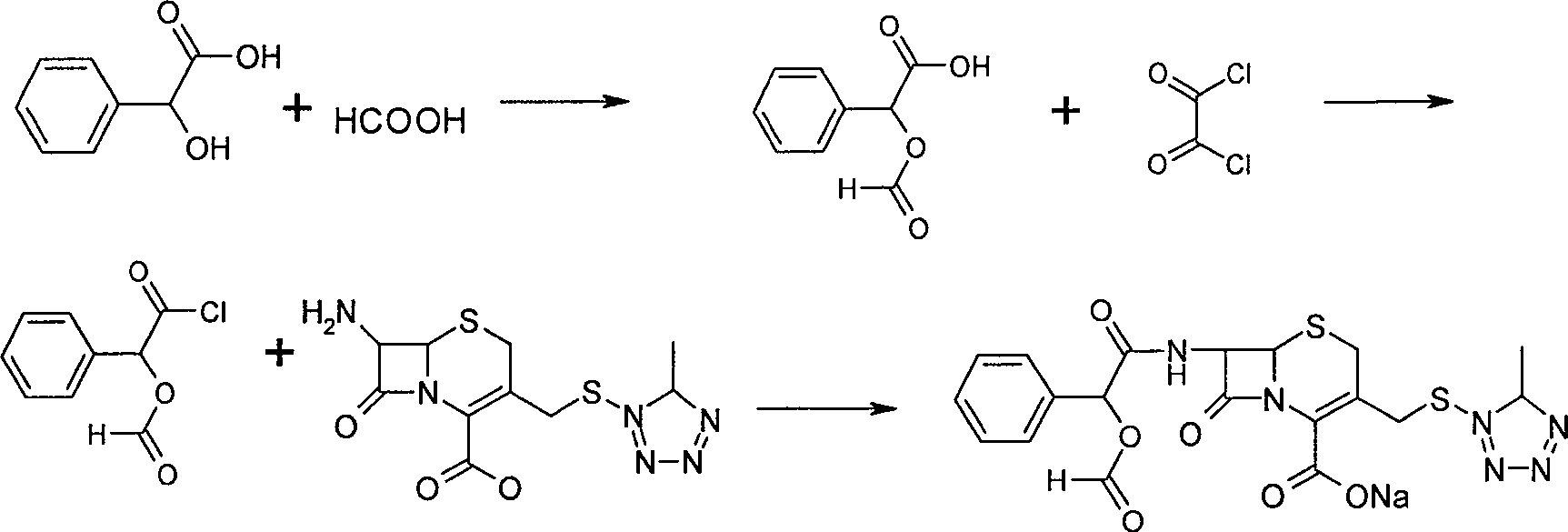
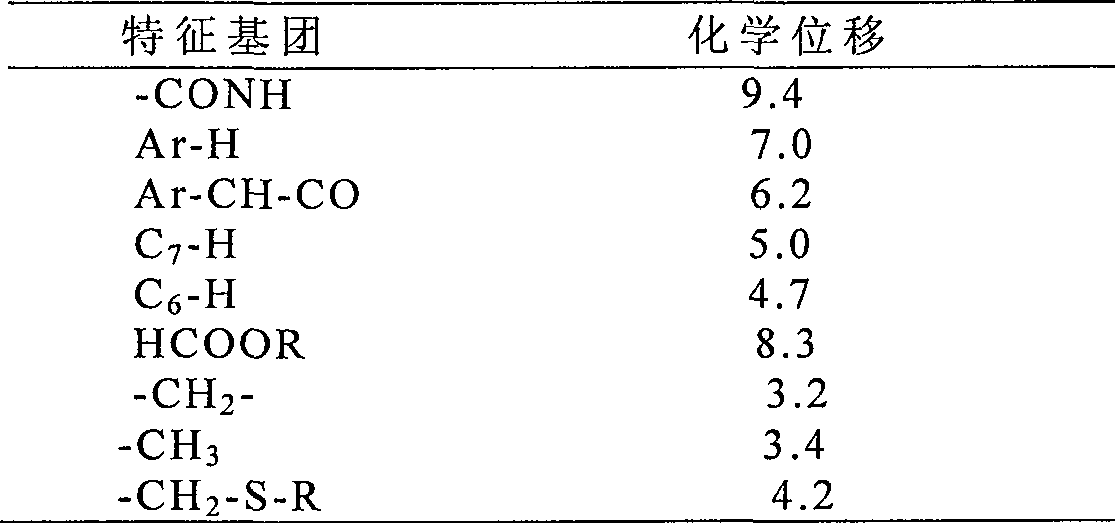
The invention relates to a synthetic method of the antibiotic cefamandole nafate, which adopts the 7-amino-3-(1-methyl-1H-tetrazoline-5-base)-sulfomethyl-3-cef-4-carboxylic acid as raw material and gains the solid of the cefamandole nafate through such five steps as the silylation reaction, acylation reaction, hydrolysis reaction, decolorization and salifying. The silanizing agent used in the silylation reaction adopts the mixture of the silicon amine alkane and the alkylogen silane. Compared with the prior art, the invention is characterized by simple in process operation, low in cost, high in product yield, good in product quality and suitable for industrial production.
Owner:苏州盛达药业有限公司
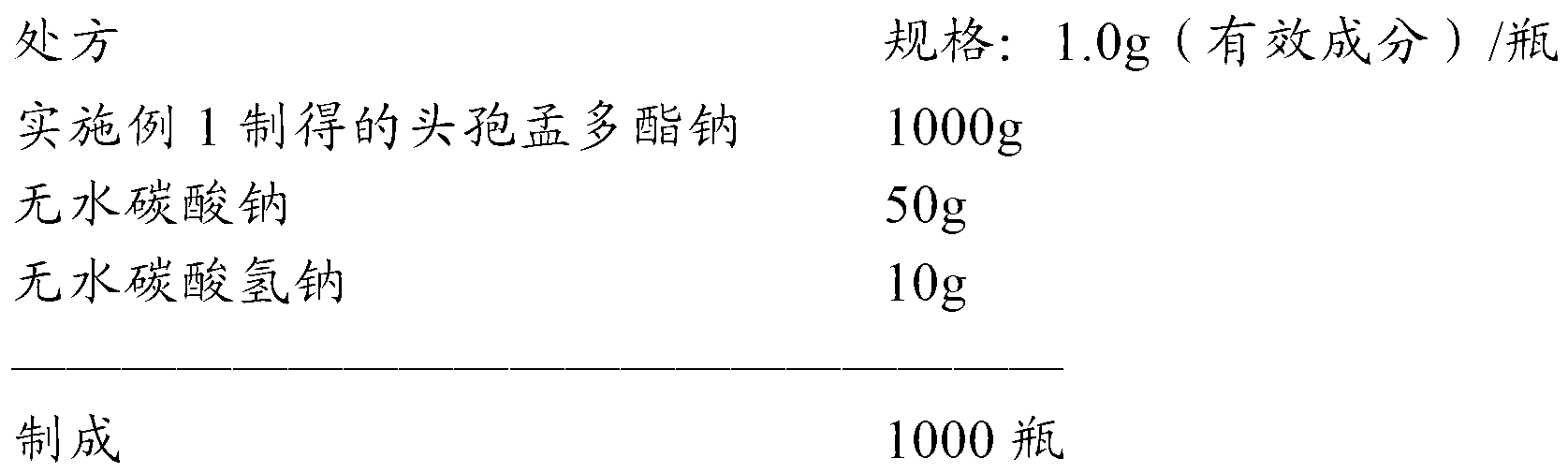
Cefamandole nafate powder injection, production method of powder injection and raw machine thereof
ActiveCN101219117AFast dissolutionHigh clarityAntibacterial agentsOrganic active ingredientsSodium carbonate anhydrousPowder injection
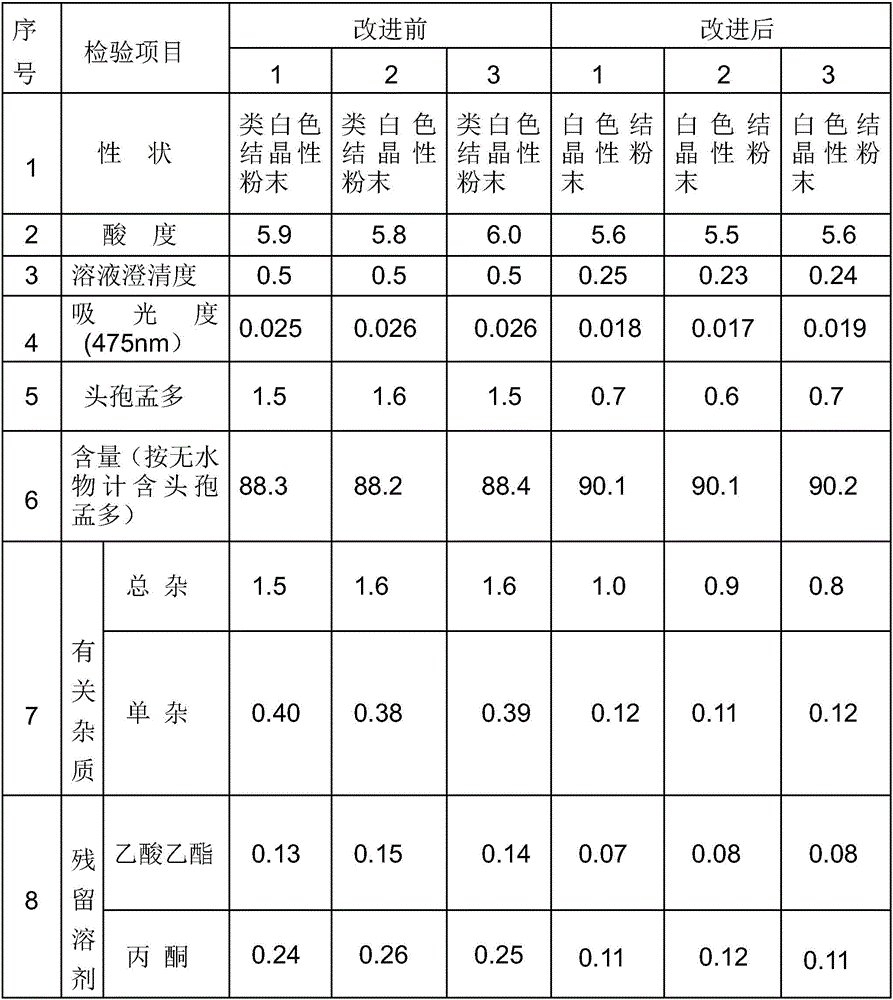
The invention relates to an aseptic powder injection of cefamandole nafate and a preparation method thereof; the aseptic powder injection of cefamandole nafate comprises cefamandole nafate and anhydrous sodium carbonate; after the improvement of the preparation technology, the dosages of auxiliary material are small, and therefore the aseptic powder injection of cefamandole nafate obtained is good in stability and low in cost. The synthetic process of the drug material of cefamandole nafate adopted by the invention has simple operation process, low material cost and moderate reaction conditions; the cefamandole nafate produced has high purity and high productive rate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
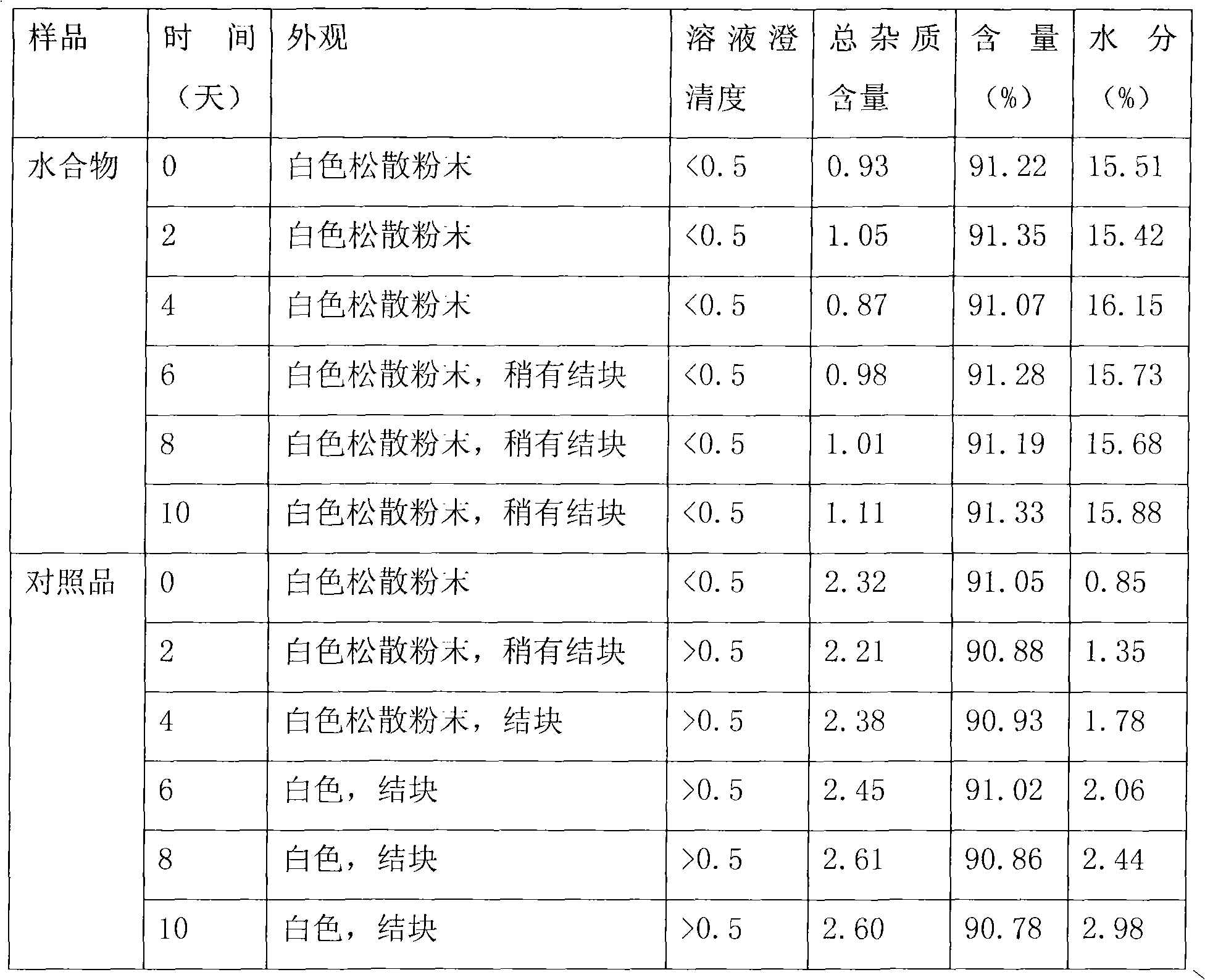
Cefamandole nafate hydrate and preparation method thereof
ActiveCN101914106AFlat surfaceImprove stabilityAntibacterial agentsOrganic active ingredientsClinical efficacyElution
The invention relates to a cefamandole nafate hydrate. The molecular formula of the cefamandole nafate hydrate is C19H17N6NaO6S25H2O. The preparation method of the cefamandole nafate hydrate comprises the following steps of: dissolving cefamandole nafate crude drug in water and placing the dissolved cefamandole nafate crude drug in a crystallizer; adding 5-15 parts of elution agents, cooling the mixture to 2-8 DEG C, separating out solids and then filtering; and rinsing the solids in the elution agents, drying for 3-5 hours at the drying temperature of 10-50 DEG C under a ventilated or vacuumcondition to obtain the cefamandole nafate hydrate. The invention also provides a cefamandole nafate for injection, which is prepared by using the cefamandole nafate hydrate as the crude drug. The cefamandole nafate hydrate obtained in the method has the advantages of smooth surface, good stability and high dissolving sped in water. After the cefamandole nafate hydrate is dissolved in the water, the action mechanism and the clinical effect of the cefamandole nafate hydrate are the same as those of cefamandole nafate prepared in the traditional process.
Owner:湖北美林药业有限公司
Separation and purification method of cefamandole nafate and preparation of cefathiamidine freeze-dried injectable powder
InactiveCN101279979AHigh purityNo pollution in the processAntibacterial agentsOrganic active ingredientsPurification methodsFreeze-drying
Disclosed is a method to separate and purify cefamandole nafate, which is characterized in that cefamandole nafate is separated and purified for three times through a high-speed countercurrent chromatograph which adopts a solvent system composed of trichloromethane, ethyl acetate, carbinol and water, with the upper phase being stationary and the lower phase being mobile. The cefamandole nafate can be further froze and dried to prepare freeze-dried powder injection. The method greatly increases the purity of the material up to 99%, and the purification process causes no pollution; therefore the method is good for industrial continuous production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method of cefamandole nafate
ActiveCN101880290AReduce generationImprove conversion rateOrganic chemistrySodium bicarbonateOrganic acid
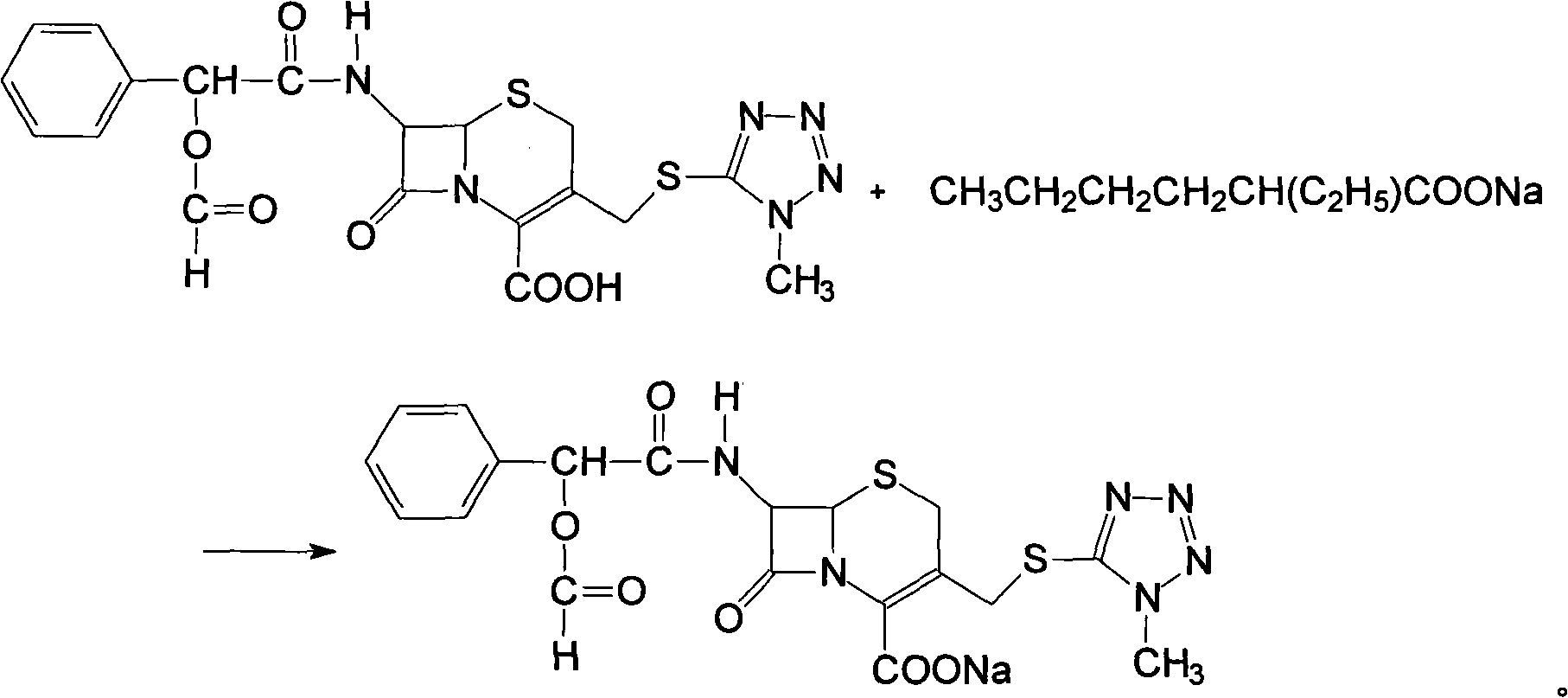
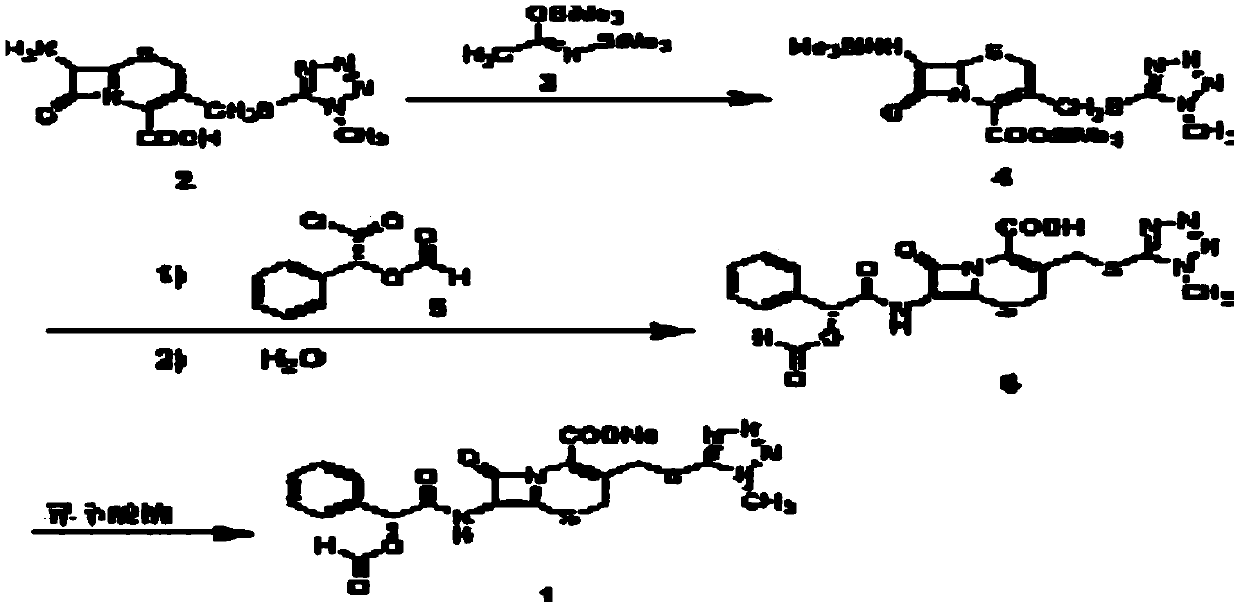
The invention discloses a preparation method of cefamandole nafate, which comprises the following steps: (1) suspending 7-amino-3-[(1-methyl-1H-tetrazol-5-yl) S-methyl] -3-cephem-4-carboxylic acid and sodium bicarbonate in an acetone water solution, adding the acetone solution of alpha-formylmandeloyl chloride to carry out a condensation reaction, and preparing 7-D-(2-formyloxy phenylacetamide)-3- [(1-methyl-1H-tetrazol-5-yl) S-methyl]-3-cephem-4-carboxylic acid; and (2) dissolving the 7-D-(2-formyloxy phenylacetamide)-3- [(1-methyl-1H-tetrazol-5-yl) S-methyl]-3-cephem-4-carboxylic acid in acetone to carry out a salification reaction with the acetone solution of organic acid sodium to prepare the cefamandole nafate. The method has the advantages of simple technology, high product yield, high purity, high reaction selectivity and use of no special equipment in production, and is suitable for industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
New method for preparing Cefamandole Nafate
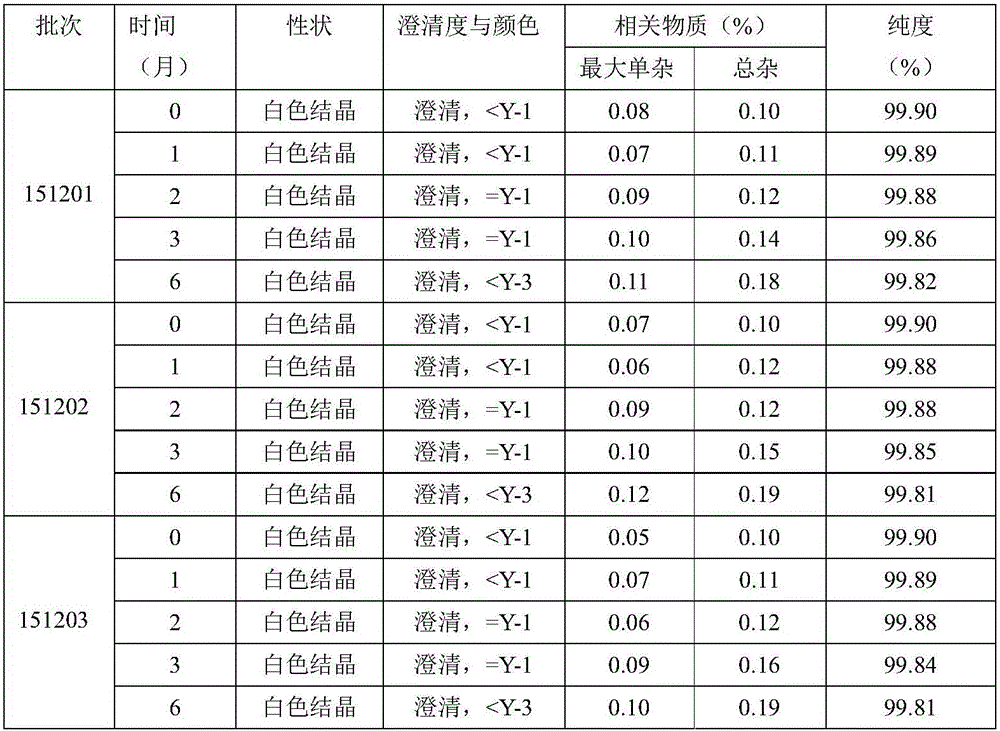
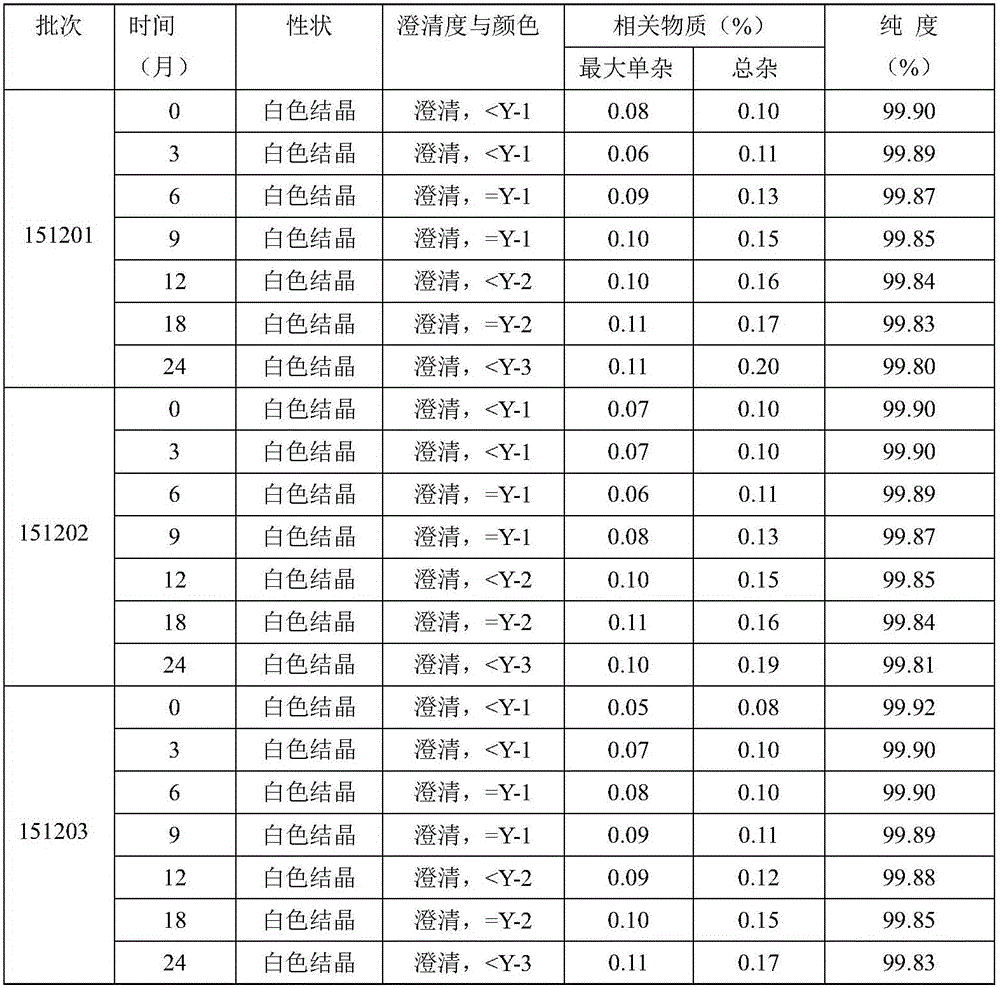
The invention relates to a new method for refining Cefamandole Nafate. The method comprises the following steps of: 1), adsorbing the Cefamandole Nafate by using a strongly acidic ion to exchange resin, performing elution, collecting eluent and concentrating under reduced pressure to obtain the Cefamandole Nafate which is primarily purified; 2) neutralizing by using sodium hydroxide aqueous solution or alkaline sodium salt aqueous solution, adjusting the pH value, and filtering under the hot condition to remove undissolved substances so as to obtain the aqueous solution of the Cefamandole Nafate which is secondarily purified; and 3) adding ethanol into the aqueous solution and recrystallizing by controlling the temperature to obtain the Cefamandole Nafate which is tertiarily purified, wherein the volume ratio of the ethanol to the water is 4:6. The Cefamandole Nafate refined products prepared by the method have purity of not less than 99.5 percent and extremely low heavy metal content, wherein the purity of most of the refined products is not less than 99.6 percent.
Owner:HAINAN LINGKANG PHARMA CO LTD
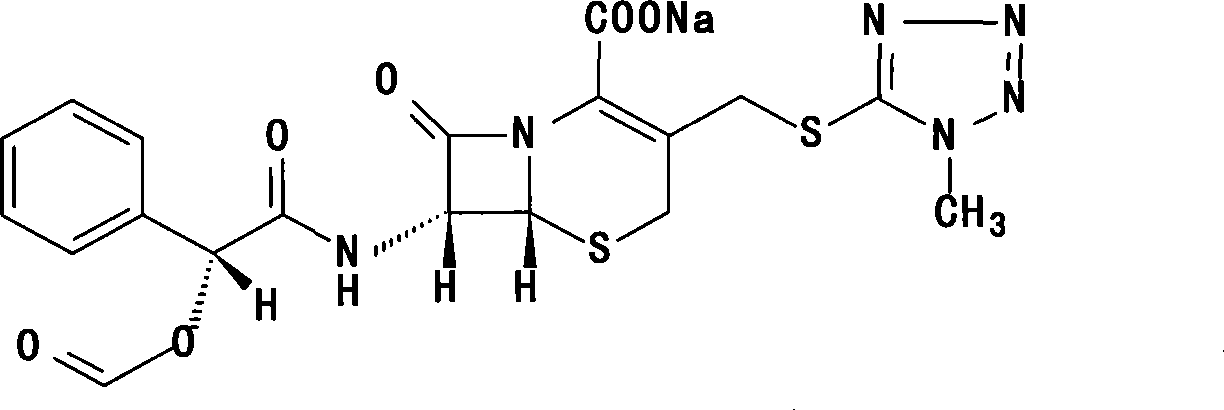
Cefamandole nafate compound and pharmaceutical composition thereof
ActiveCN103044453AImprove stabilityLess insoluble particlesAntibacterial agentsOrganic active ingredientsChemical compoundPharmaceutical Substances
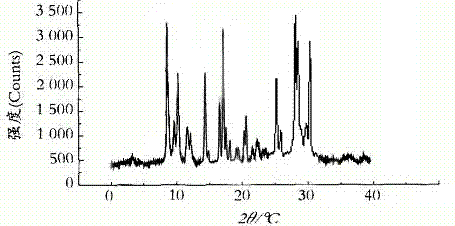
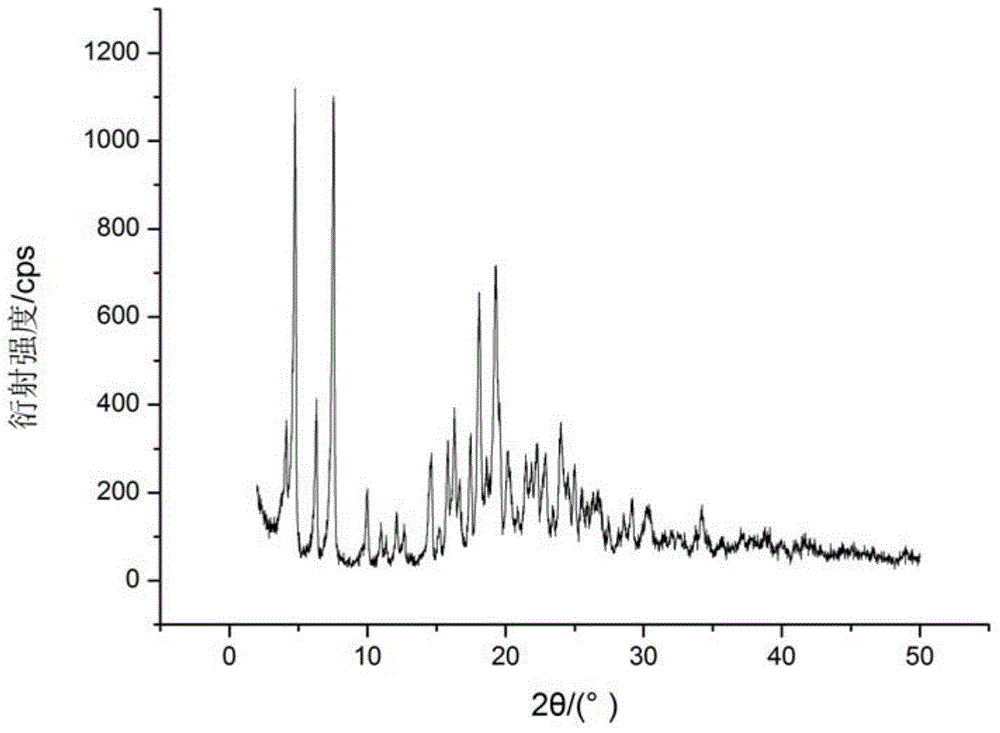
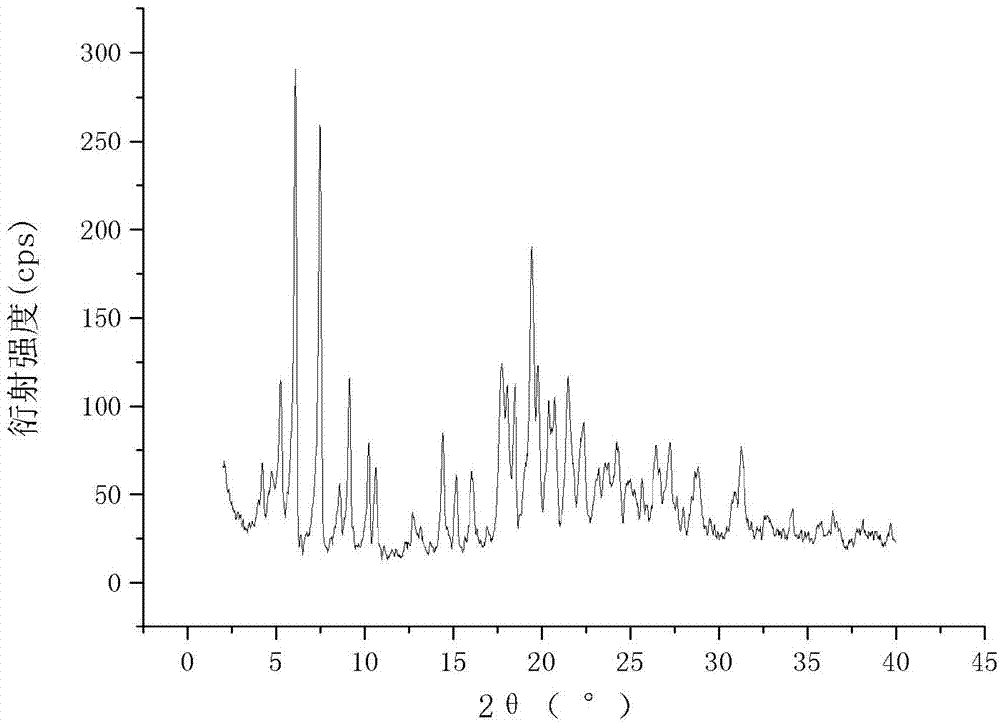
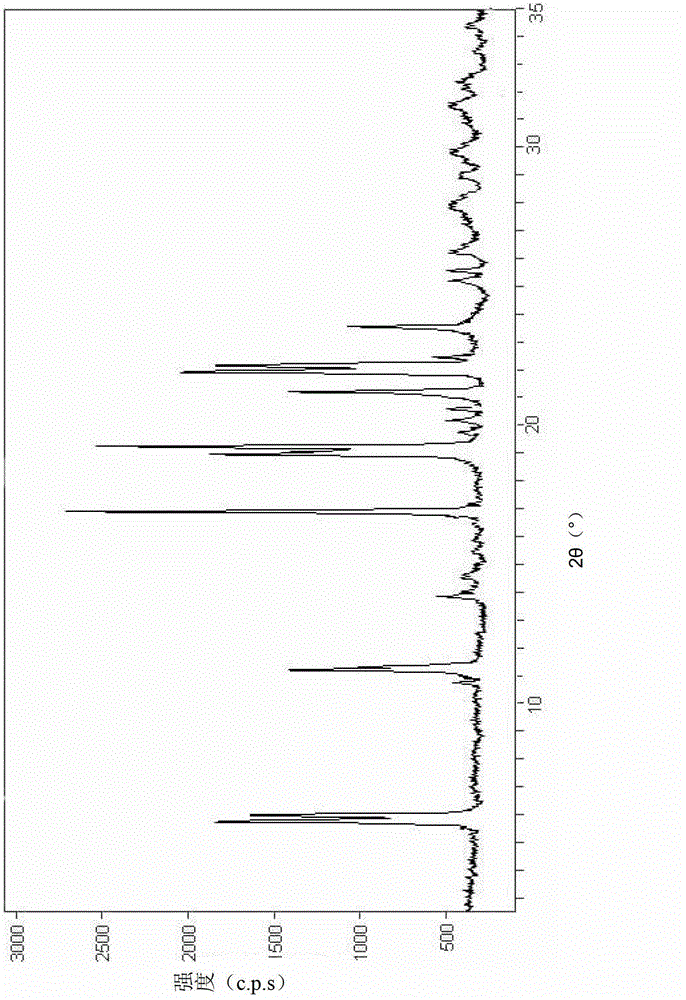
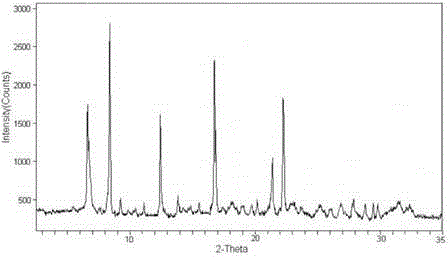
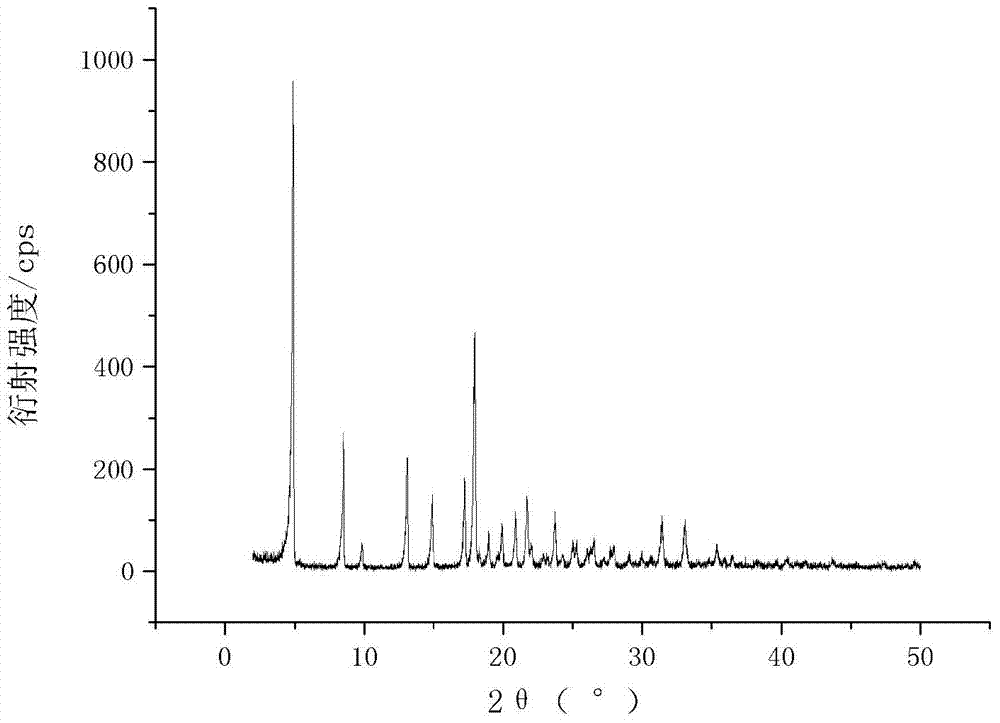
The invention relates to a cefamandole nafate compound and a pharmaceutical composition of the cefamandole nafate compound. The cefamandole nafate compound is crystal, and is determined by X-ray powder diffraction, and the characteristic peak in an atlas is shown as 8.8 degrees, 9.5 degrees, 10.2 degrees, 11.3 degrees, 12.0 degrees, 14.4 degrees, 16.8 degrees, 17.3 degrees, 20.6 degrees, 25.0 degrees, 27.8 degrees, 28.1 degrees and 30.2 degrees in a range of 20+ / 0.2 degrees. The pharmaceutical composition of the cefamandole nafate compound is a preparation and a pharmaceutical composition preparation containing the above cefamandole nafate compound and the preparation is powder-injection. The cefamandole nafate crystalline compound is high in stability and few in insoluble particles; a cosolvent is not needed, so that the compound can be directly and aseptically sub-packaged to obtain cefamandole nafate powder injection for injection. The cefamandole nafate crystalline compound is also fully mixed with aseptic sodium carbonate powder to be aseptically sub-packaged. The number of insoluble particles added with the powder injection of sodium carbonate is few on the basis of reaching standard request.
Owner:HUNAN KELUN PHARMA
Method for preparation of fine cefamandole nafate
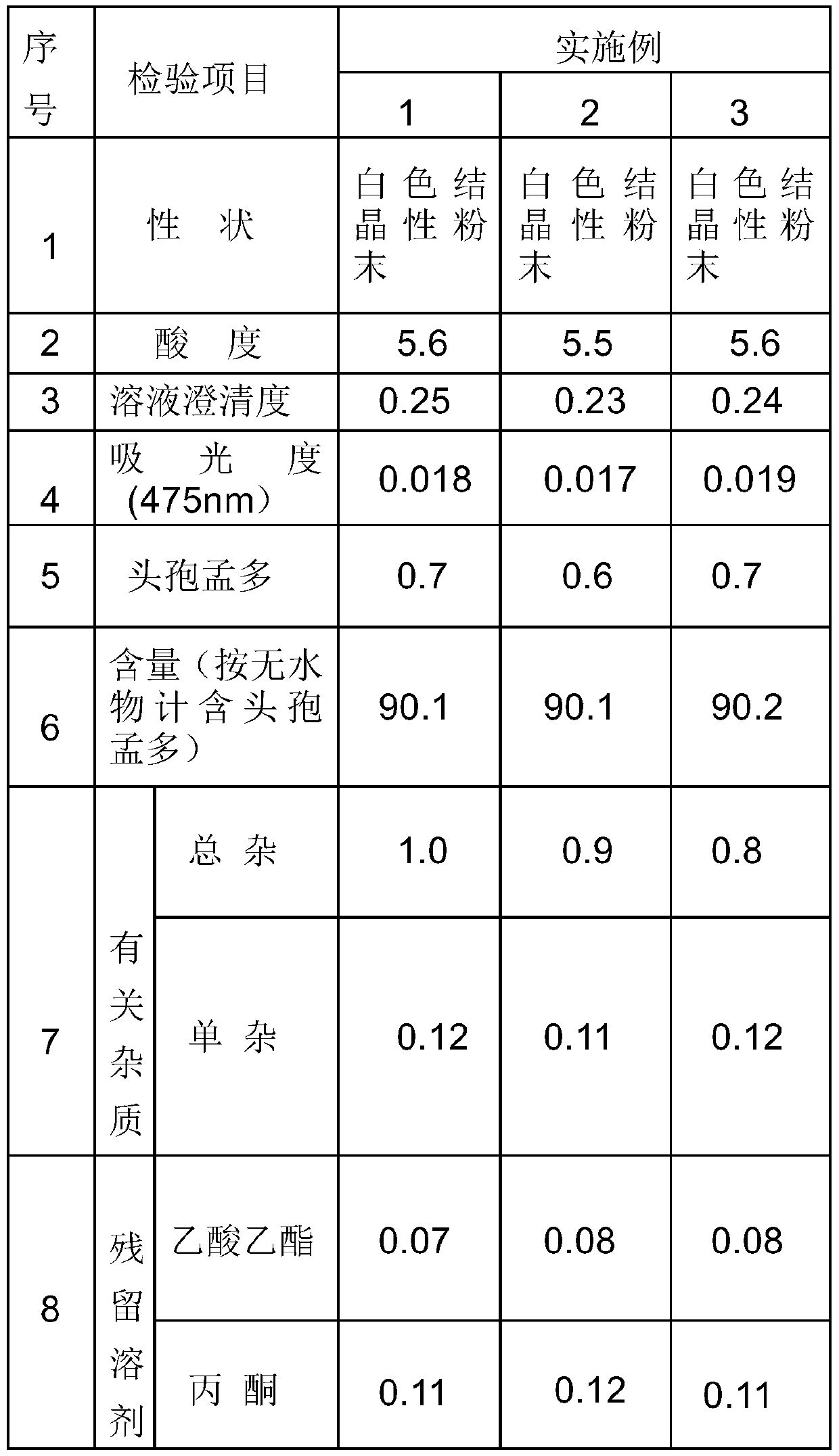
The invention discloses a method for preparation of fine cefamandole nafate, comprising the steps of mixing crude cefamandole nafate with organic sodium salt, salt-free water and organic solvent (1); controlling pH value of the reaction system to 5.5-7.5 and reaction temperature to 5-15 DEG C; adding active carbon and then filtering to obtain filtrate; mixing the filtrate with organic solvent (2) and separating out crystal to obtain the cefamandole nafate. The method for preparation of the fine cefamandole nafate provided by the invention overcomes the disadvantages of prior preparation method, and has the advantages of simple operation, short preparation period, easy quality control, good product stability and important application value.
Owner:SHANDONG LUKANG PHARMA
Preparation process of cefamandole nafate
The invention discloses a preparation process of cefamandole nafate. The preparation process comprises steps of: heating and stirring 7-amino cephalosporanic acid, 5-mercapto-1-methyltetrazole and a catalyst boron trifluoride acetonitrile complex for a reaction; and carrying out a cooling post-treatment to obtain cefditoren nuclear parent; conducting a heating reflux reaction on the cefditoren nuclear parent and a silanizing agent until the solution turns to a clarified state; adding N, N-dimethyl aniline under the protection of inert gas at a low temperature, dropwise adding D-(-)-O-formyl mandeloyl chloride for reaction, and carrying out post-treatment to obtain formyl cefamandole acid; and reacting the formyl cefamandole acid with an organic acid salt, and recrystallizing to obtain the cefamandole nafate. By the above way, the preparation process of cefamandole nafate provided by the invention employs a simple and easily implemented process to obtain high-yield cefditoren nuclear parent with low impurity content; dichloromethane is used as a solvent to obtain the cefamandole acid with greatly enhanced color grade and yield; and dosage of activated carbon in the post-treatment is obviously reduced, so as to reduce the production cost.
Owner:苏州盛达药业有限公司
Method for refining cefamandole nafate, cefamandole nafate and application thereof
ActiveCN103073562AReduce contentHigh purityAntibacterial agentsOrganic active ingredientsAcetic acidDevitrification
The invention provides a method for refining cefamandole nafate, which comprises the following steps: (1) mixing lower alcohol and acetone or mixing lower alcohol and ethyl acetate to obtain a mixed solvent, wherein the volume ratio of the lower alcohol to the acetone or that of the lower alcohol to the ethyl acetate is (1-2) : (5-10); (2) dissolving the crude cefamandole nafate in a stirring manner into water for injection, and adjusting pH to 6-8 to obtain a crude solution; (3) when stirring, dropwise adding the mixed solvent into the crude solution, and then, dropwise adding ethyl acetate or acetone with the volume 30-40% of that of the mixed solvent, so as to obtain a suspension; and (4) cooling the suspension to 3-5 DEG C, standing for devitrification for 12-36 hours, vacuum filtration and drying to obtain refined cefamandole nafate. The invention further provides cefamandole nafate produced according to the refined method, sterile composition powder-injection preparation containing the cefamandole nafate, and application of the cefamandole nafate in preparing antibacterial agents.
Owner:HAIKOU PHARMA FACTORY +1
Novel crystal form of cefamandole nafate compound and crystal preparation method thereof
ActiveCN104530086AEasy to joinEasy to operateAntibacterial agentsOrganic chemistry methodsOrganic solventX-ray
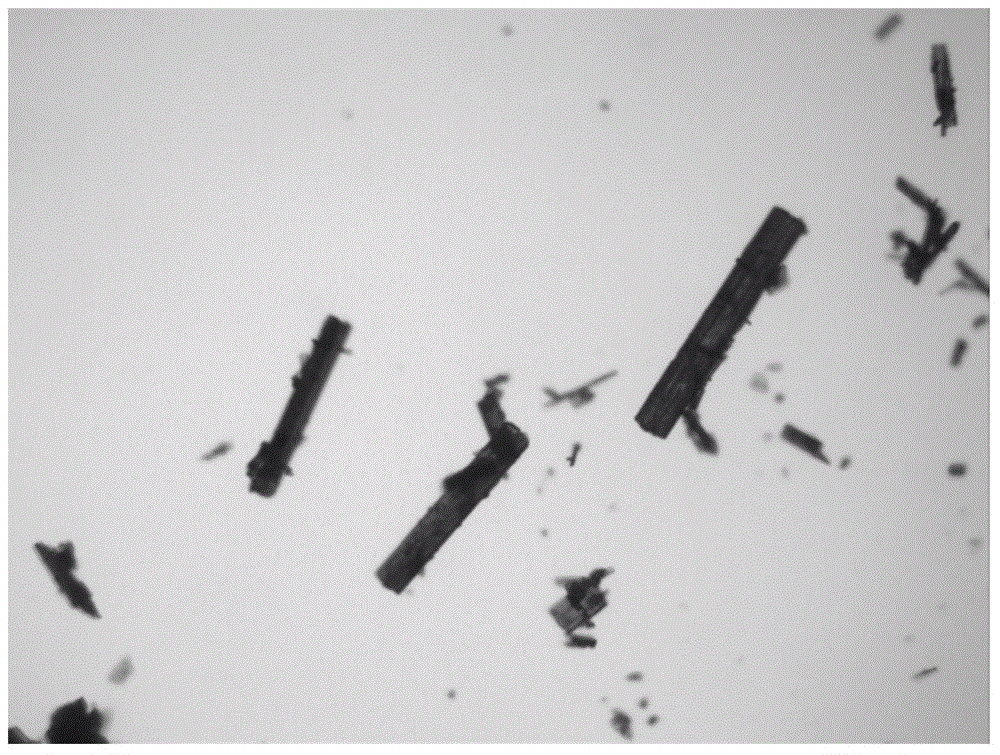
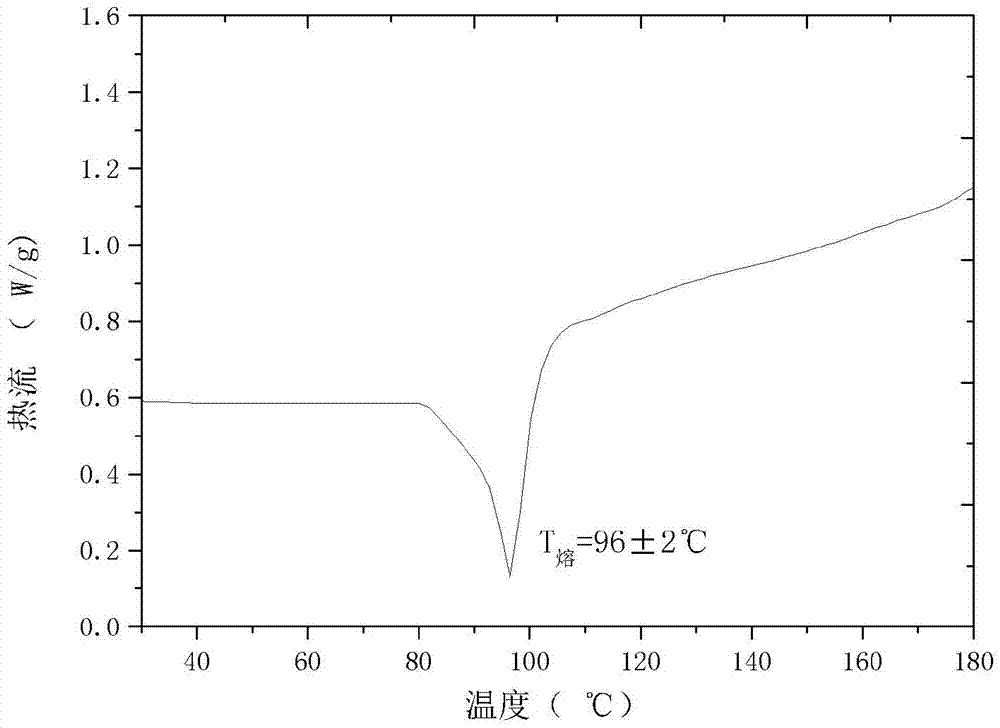
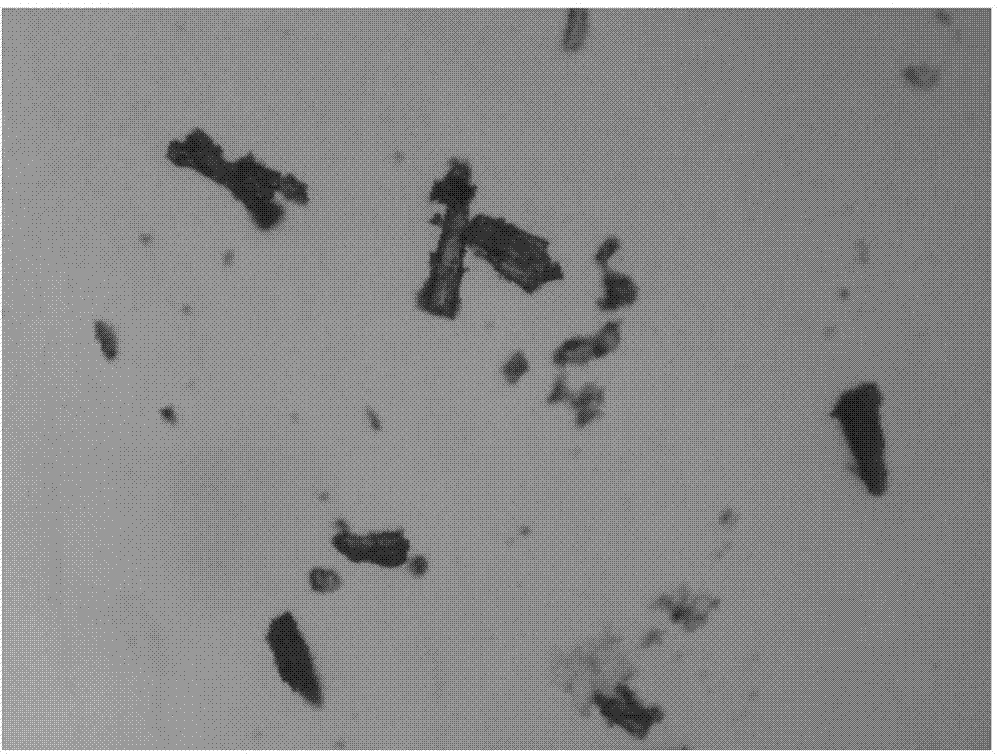
The invention relates to a novel crystal form of a cefamandole nafate compound and a crystal preparation method thereof. Characteristic peaks at diffraction angles 2theta and DSC are defined by using an X-ray powder diffraction map. The preparation method of crystals with novel crystal form of the cefamandole nafate compound comprises the following steps: adding a cefamandole nafate solid into an organic solvent to prepare a 0.04-0.3g / ml suspension; stirring and suspending for a while at 40-50 DEG C; then, cooling to 5-15 DEG C at a certain cooling rate for a while; carrying out suction filtration on the suspension to obtain a filter cake which is a cefamandole nafate wet product; and drying the cefamandole nafate wet crystal product to constant weight so as to obtain a final novel crystal form product of the cefamandole nafate compound. The novel crystal form product has good thermal stability and is hardly deteriorated when being stored. Meanwhile, the novel crystal form is in a shape of a thick rod and has good mobility and high bulk density, so that the packaging and transporting convenience of the product is remarkably improved.
Owner:TIANJIN UNIV +1
Method for preparing cefamandole nafate powder injection preparation
The invention provides a method for preparing a cefamandole nafate powder injection preparation. The method comprises the following steps: (a) performing a silanization reaction on 7-ACT and a silanization agent in a dichloromethane solvent under temperature control, and reducing the temperature after the reaction is completed so as to obtain a reaction liquid 1, wherein the solid-liquid ratio of7-ACT to dichloromethane is 1g:(3-6)ml; (b) dropwise adding (R)-(-)-O-formylmandeloyl chloride into the reaction liquid 1, controlling the temperature, performing an acylation reaction so as to obtaina reaction liquid 2; (c) performing treatment of extraction, decoloring and dehydration on the reaction liquid 2; (d) performing temperature control crystallization; and (e) washing a solid with acetone, drying, performing sterile sub-packaging, thereby obtaining the cefamandole nafate powder injection preparation. Due to adoption of a high-concentration dichloromethane reaction system, the method is rapid in silanization and acylation process, short in reaction time, low in reaction residue, high in product yield and small in impurity. After the reactions, organic solvents such as dichloromethane can be easily recycled and repeatedly used, so that the method is relatively environment-friendly, low in cost and applicable to large-scale industrial production.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Cefamandole nafate new crystal form and crystallization preparing method thereof
The invention relates to a cefamandole nafate new crystal form and a crystallization preparing method thereof. The cefamandole nafate new crystal form is characterized by characterizing the characteristic peaks of a diffraction angle 2 theta degree and a DSC by using X-ray powder diffraction patterns, wherein the cefamandole nafate new crystal form is defined as VI. The preparing method comprises the following steps of adding the cefamandole nafate solid into the good solvent, and mixing at the temperature of 10-30 DEG C to enable the cefamandole nafate to dissolve completely, wherein the solution concentration is 0.05-0.4 g / ml; adding additives at different concentrations into the solution; adding a solventing-out agent into the solution, wherein the dosage of the solventing-out agent is 5-20 times the dosage of the good solvent; after cultivating the crystal, performing suction filtration on formed suspension liquid; drying to obtain a cefamandole nafate new crystal form product. The solubility of the new crystal form is improved by more than 5%; the new crystal form has higher solubility, so that the dissolution rate of a medicinal preparation can be improved. The traditional stable crystal form is of a needle shape, but the appearance of the new crystal form is of a prism shape, thus, the bulk density of products is improved by more than 8%, better mobility is realized, and accordingly convenience in package and transport of the products is improved.
Owner:TIANJIN UNIV +1
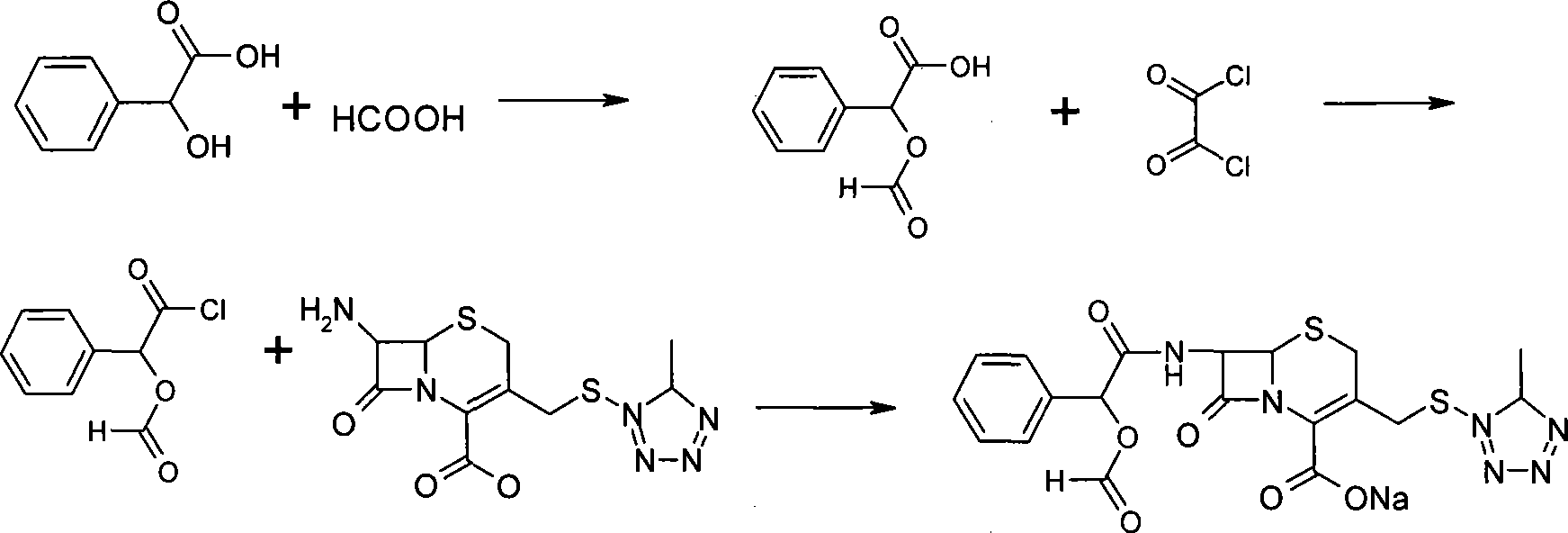
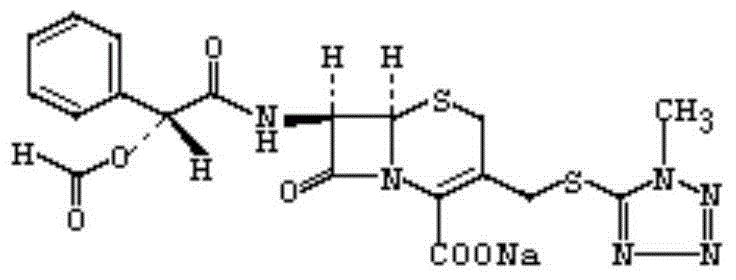
Synthesis method for dextrorotation cefamandole nafate
The invention discloses a synthesis method for dextrorotation cefamandole nafate. The method includes the steps that in one-element organic solvent, 7-ACA and 1-methyl-1h-tetrazole-5-thiol are subjected to condensation with a boron trifluoride complex of the one-element organic solvent as a catalyst so as to generate 7-ATCA; and then, in another one-element organic solvent, under the effect of organic alkali, the 7-ATCA and S-(-)-formylmandeloyl chloride are made to react so as to generate the dextrorotation cefamandole nafate, then, an organic phase is left after hydrolysis and extraction phase splitting, organic acid sodium salt and another organic solvent indissolvable in dextrorotation cefamandole sodium are added, the dextrorotation cefamandole sodium is obtained through crystallization, finally, the dextrorotation cefamandole sodium is dissolved in water, organic solvent incapable of being dissolved in water is added together with organic or inorganic acid, an organic phase is left after extraction phase splitting, and reduced pressure rotary evaporation is conducted on the organic phase to obtain the dextrorotation cefamandole nafate. The method is simple, conditions are gentle, raw materials are easy to obtain, and purity of the finally-obtained product is 99% or above.
Owner:哈药集团股份有限公司 +1
Separation and purification method of cefamandole nafate and preparation of cefathiamidine freeze-dried injectable powder
InactiveCN101279979BHigh purityNo pollution in the processAntibacterial agentsOrganic active ingredientsPurification methodsFreeze-drying
Disclosed is a method to separate and purify cefamandole nafate, which is characterized in that cefamandole nafate is separated and purified for three times through a high-speed countercurrent chromatograph which adopts a solvent system composed of trichloromethane, ethyl acetate, carbinol and water, with the upper phase being stationary and the lower phase being mobile. The cefamandole nafate can be further froze and dried to prepare freeze-dried powder injection. The method greatly increases the purity of the material up to 99%, and the purification process causes no pollution; therefore the method is good for industrial continuous production.
Owner:HAINAN LINGKANG PHARMA CO LTD
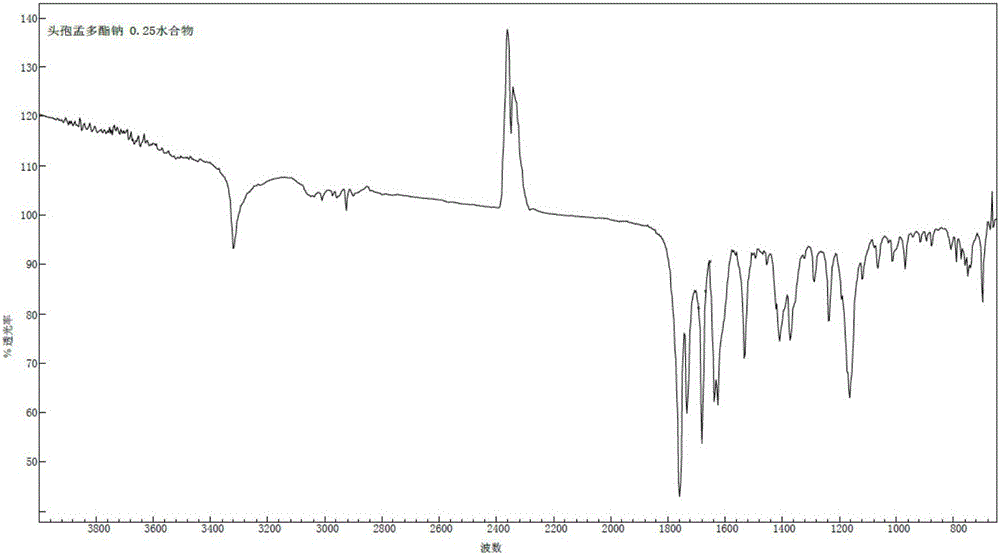
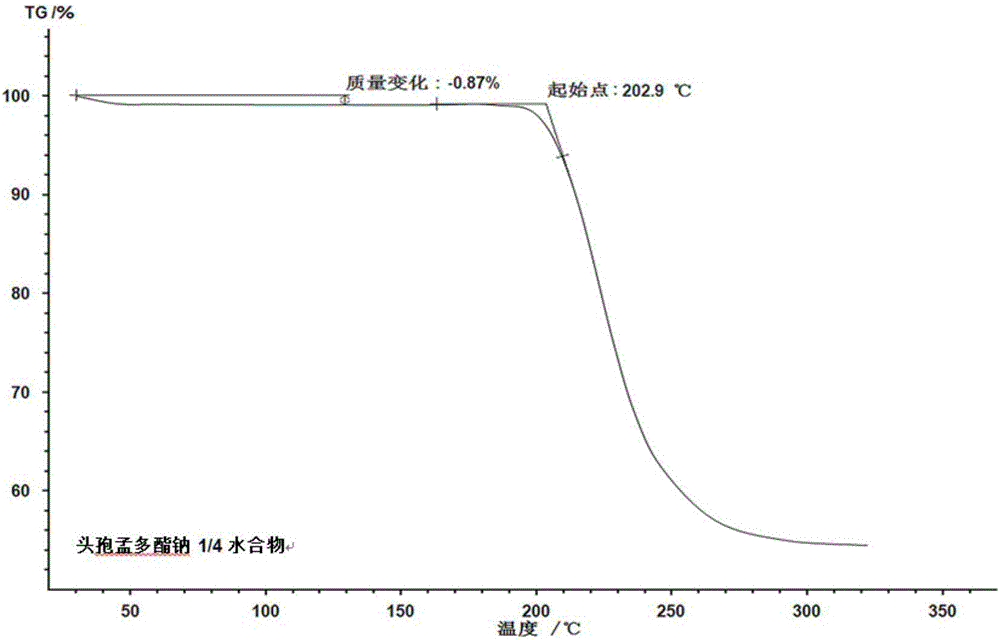
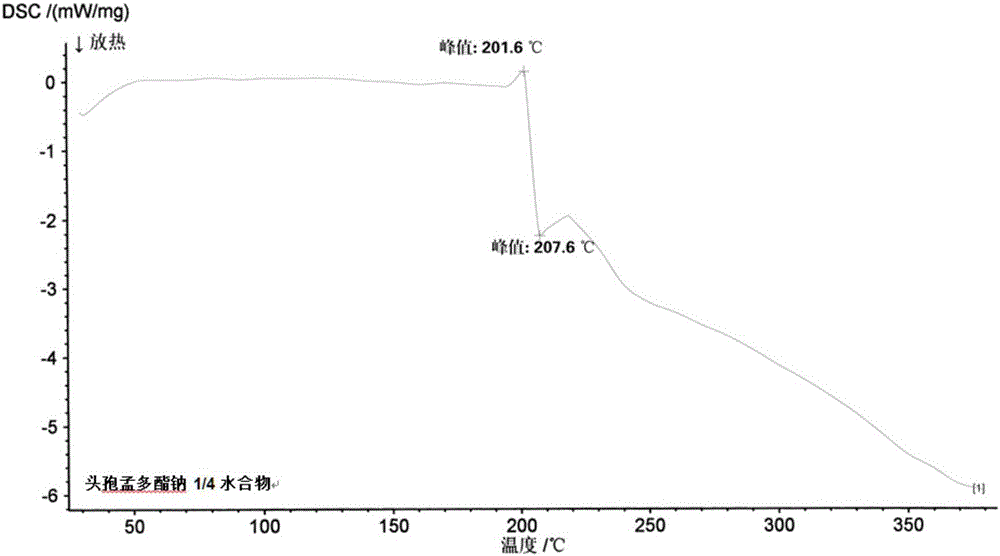
1/4 water and cefamandole nafate compound
ActiveCN106831821AHigh yieldLow costAntibacterial agentsOrganic active ingredientsImpurityCefamandole nafate
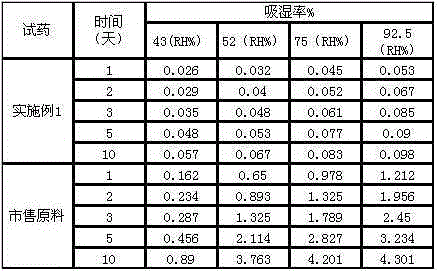
The invention discloses a 1 / 4 water and cefamandole nafate compound and a preparation method thereof. Each mole of cefamandole nafate contains 1 / 4 mole of water. The cefamandole nafate compound prepared by the method is low in impurity content, good in stability, good in mobility and low in hygroscopicity, and has a wider application prospect.
Owner:HAINAN LINGKANG PHARMA CO LTD
Suspension powder injection of cefamandole nafate and new application thereof
InactiveCN101637456AEasy to storeEasy to transportAntibacterial agentsPowder deliverySuppurative cholangitisSURFACTANT BLEND
The invention relates to suspension powder injection of cefamandole nafate and a preparation method thereof and further relates to new application of the injection in treating acute suppurative cholangitis. The frozen-dried suspension powder injection comprises the following components in parts by weight: 1 part of cefamandole nafate, 1.5-12 parts of surfactant and 2-15 parts of frozen-dried supporting agents.
Owner:HAINAN YONGTIAN PHARMA INST
Medicine composite containing cefamandole nafate compound
InactiveCN103145736AImprove stabilityImprove liquidityAntibacterial agentsPowder deliveryBottlePowder injection
The invention discloses a cefamandole nafate compound for injection. The cefamandole nafate compound is a crystal compound; and in X-ray powder diffraction, main peaks are shown at diffraction angles of 5.9+ / -0.01 degrees, 6.02+ / -0.01 degrees, 11.3+ / -0.01 degrees, 16.9+ / -0.01 degrees, 17.9+ / -0.01 degrees, 18.0+ / -0.01 degrees, 21.1+ / -0.01 degrees, 22.0+ / -0.01 degrees, 22.1+ / -0.01 degrees, and 23.5+ / -0.01 degrees. The invention also discloses a medicine composite containing the cefamandole nafate compound. The cefamandole nafate crystal is good in stability and high in fluidity; when a cefamandole nafate sterile powder injection is prepared, the sub-package amount of active medicine components is accurate and the operation is facilitated; and besides, the sterile powder injection is not easily adhered to bottles, and is very convenient regardless of being used independently or mixed with other medicine powder. The cefamandole nafate compound does not degrade fundamentally and can be placed for a long time, thereby having a great advantage in medicine preparation.
Owner:四川省惠达药业有限公司
Preparation method of Cefamandole Nafate powder injection for injection
InactiveCN106511281AQuality improvementTo achieve the effect of decolorizationAntibacterial agentsOrganic active ingredientsCentrifugationFiltration
The invention discloses a preparation method of a Cefamandole Nafate powder injection for injection. The method includes the steps of: (1) strain preparation; (2) preparation and culture of seed tank; (3) preparation and culture of a fermentation tank; (4) filtration; (5) decolorization and concentration; (6) preparation of a reaction tank and pipeline; (7) preparation of enzyme catalytic reaction; (8) enzyme catalytic reaction; (9) crystallization; (10) centrifugation and vacuum drying; (11) preparation of 7-ATCA; (12) preparation of cefamandole acid; (13) preparation of Cefamandole Nafate; and (14) aseptic subpackaging, thus obtaining the Cefamandole Nafate powder injection for injection. The invention provides the method of improving the 7-ACA preparation process so as to improve the product quality of cefamandole.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Method for preparing cefamandole nafate powder preparation for injection
InactiveCN106562972AQuality improvementControl drop timeAntibacterial agentsOrganic active ingredientsDimethylaniline N-oxideChloride
The invention discloses a method for preparing a cefamandole nafate powder preparation for injection, wherein the method comprises the following steps: 1, dissolving N,N-dimethyl aniline; 2, dissolving D-formyl mandelic acid chloride; 3, carrying out a silylation reaction; 4, synthesizing cefamandole acid ester; 5, extracting; 6, carrying out dehydration and decolorization; 7, crystallizing; and 8, carrying out aseptic subpackaging. The reaction efficiency and the appearance quality of the product are improved by changing feeding and crystallization ways.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Medicine cefamandole nafate composition for treating bacterial infection
InactiveCN105055420AHigh purityEasy to prepareAntibacterial agentsOrganic active ingredientsPharmaceutical drugMicrobiology
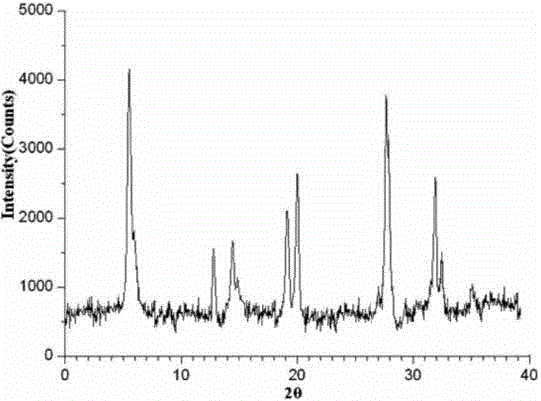
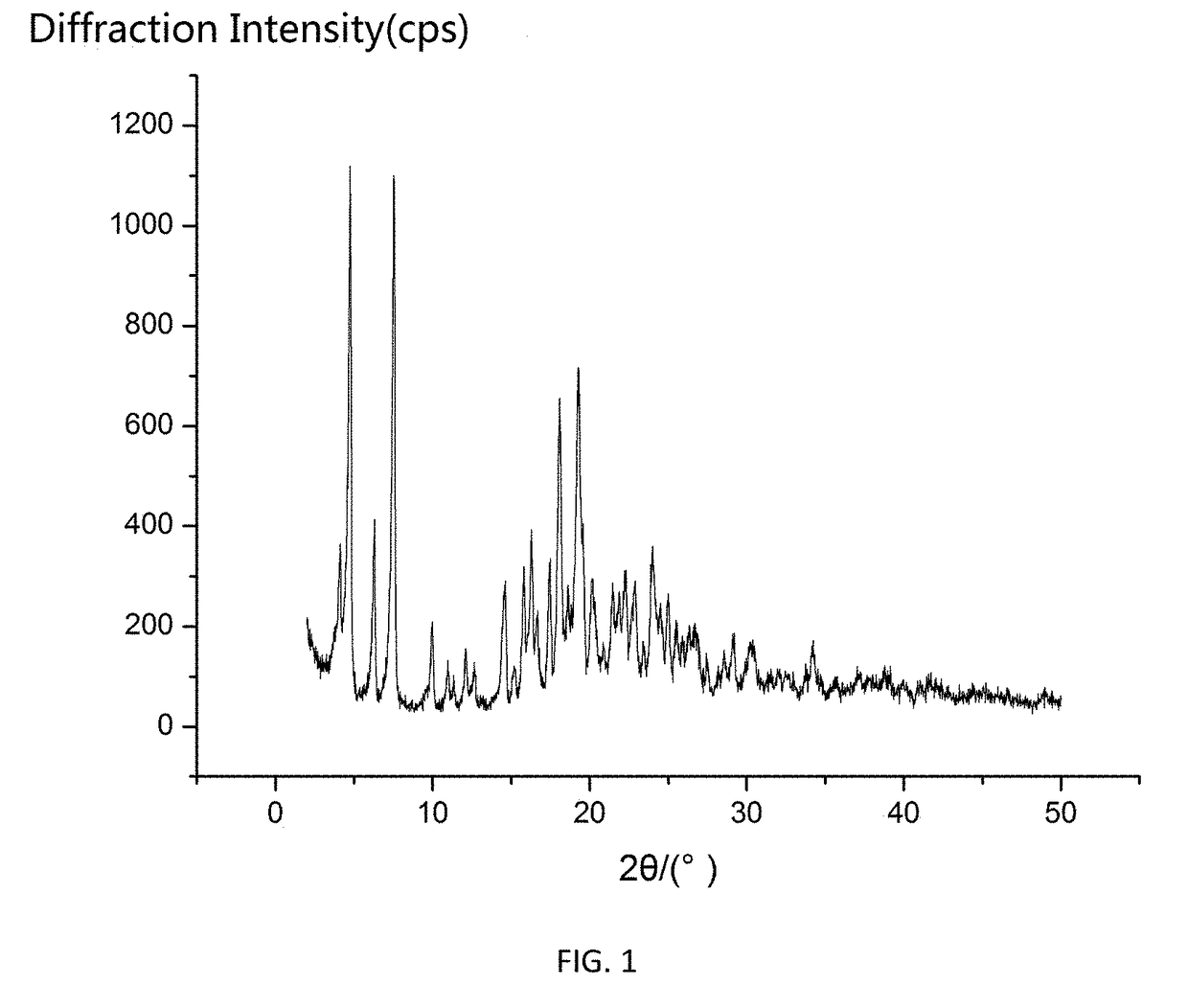
The invention relates to a cefamandole nafate medicine composition for treating bacterial infection and belongs to the technical field of medicines. The composition is composed of cefamandole nafate and sodium dihydrogen phosphate; cefamandole nafate is a crystal and an X-ray powder diffraction pattern obtained through measurement of Cu-K(alpha) ray is shown in the graph I. The novel crystal form of cefamandole provided by the invention is different from the crystal form structure in the prior art, through experimental verification, the fact that the novel crystal form compound is high in purity, good in mobility and stability, low in polymer content, low in hygroscopicity, and safe and reliable during clinical application is found, powder-injection prepared from the novel crystal form compound is good in stability, good in stability after being combined with a solvent, and extremely low in content of insoluble particles and is particularly suitable for being applied clinically.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
Cefamandole nafate composition for the treatment of infectious diseases
InactiveCN105055419AImprove stabilityLow insoluble particulate contentAntibacterial agentsOrganic active ingredientsCrystal structureInfective disorder
The present invention discloses a cefamandole nafate composition for the treatment of infectious diseases and belongs to the technical field of medicine. The composition comprises cefamandole nafate and anhydrous sodium carbonate; the cefamandole nafate is a novel crystalline form compound; and an X-ray powder diffraction pattern obtained by Cu-K alpha radiation measurement is shown as a figure 1. The cefamandole nafate novel crystalline form provided by the invention differs from the crystal structure in the prior art; experiment verification is pleasantly surprised to find that the novelcrystalline compound has high purity, good liquidity, good stability, low polymer content and no hygroscopicity, and is safe and reliable for clinical applications; powder prepared from the novel crystalline form compound has good stability, good compatibility stability with the solvent and very low content of insoluble particles, and is very suitable for clinical application.
Owner:QINGDAO LANSHENGYANG PHARMA & BIOTECH CO LTD
Novel industrial crystallization technology for cefamandole nafate
ActiveCN104892637APromote enrichmentSimple crystallization processOrganic chemistryBulk chemical productionSolventImpurity
The invention discloses a novel industrial crystallization technology for cefamandole nafate. Recrystallization of the cefamandole nafate is realized through combination of a supercritical fluid extraction technology and a traditional crystallization technology. In a whole crystallization system, extraction, adsorption, crystallization and drying processes are finished under the coaction of a supercritical fluid, a solvent, an extraction pool and a crystallization pool under specific temperature and pressure conditions, and recrystallization of the cefamandole nafate is realized. According to the method, the separation efficiency is high, the product purity is high, few impurities exist, and the preparation product quality is greatly improved.
Owner:HAINAN LINGKANG PHARMA CO LTD +1
Method for preparing cefamandole nafate sodium powder injection preparation for injection
InactiveCN110302202AQuality improvementSynthetic reaction is sufficientAntibacterial agentsOrganic active ingredientsDimethylaniline N-oxideChloride
The invention discloses a method for preparing a cefamandole nafate sodium powder injection preparation for injection. The method comprises the following steps: (1) dissolution of N,N-dimethylaniline;(2) dissolution of D-formylmandeloyl chloride; (3) silanization reaction; (4) synthesis of cefamandole acid esters; (5) extraction; (6) dehydration and decoloration; (7) crystallization; and (8) sterilized shipment. By changing the modes of charging and crystallization, the reaction efficiency and the product appearance quality are improved.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Cefamandole nafate new crystal form and crystallization preparing method thereof
InactiveCN104844626AEasy to joinImprove thermal stabilityAntibacterial agentsOrganic chemistryX-rayRoom temperature
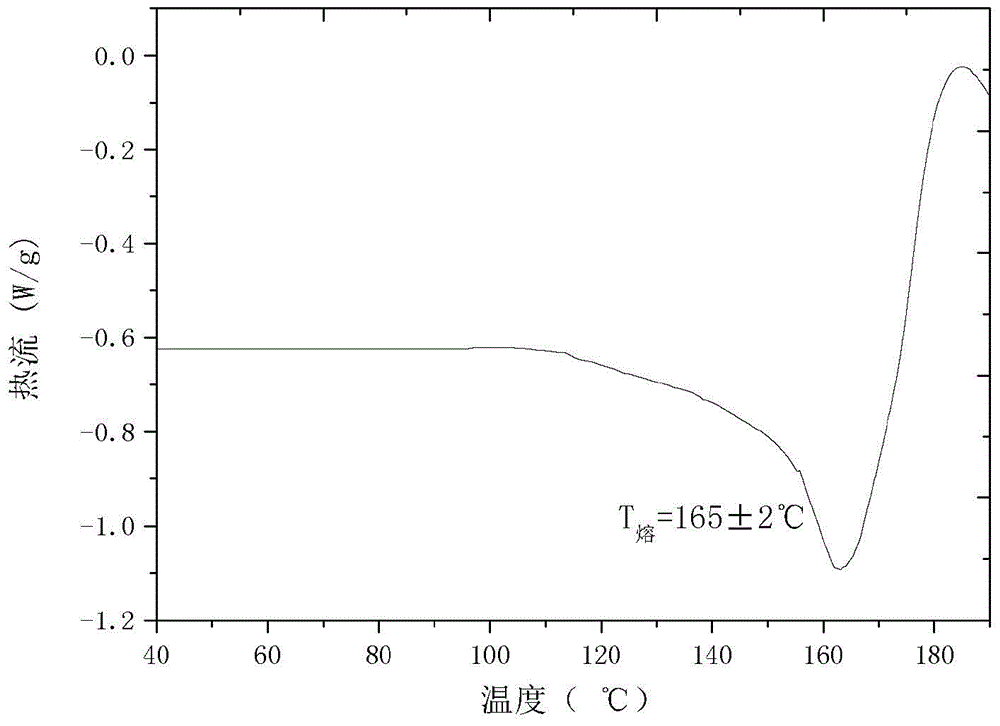
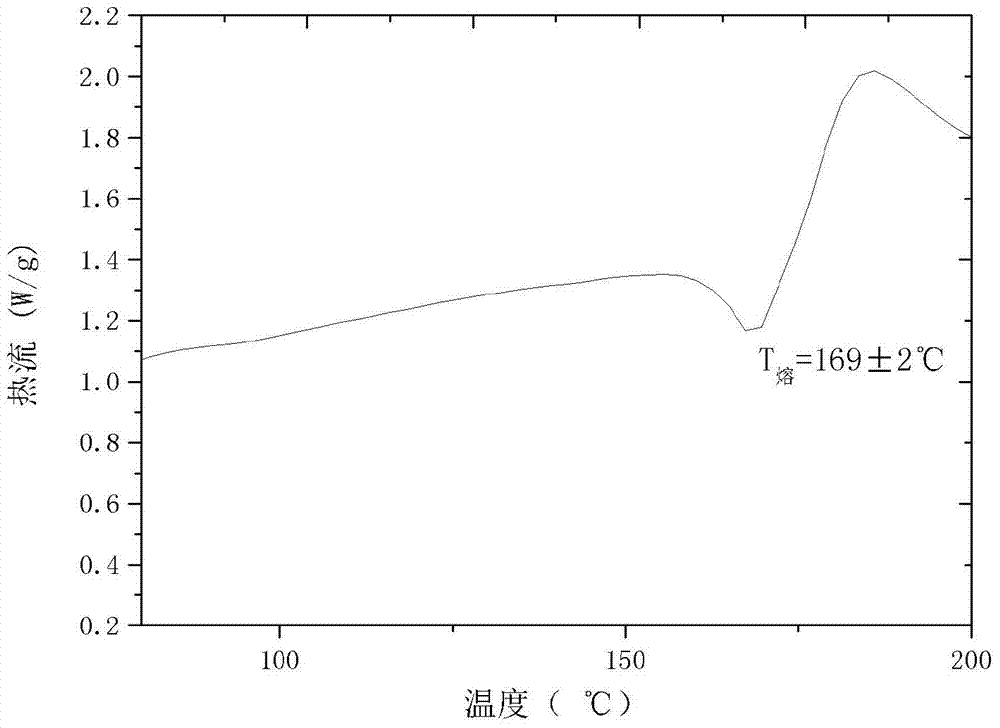
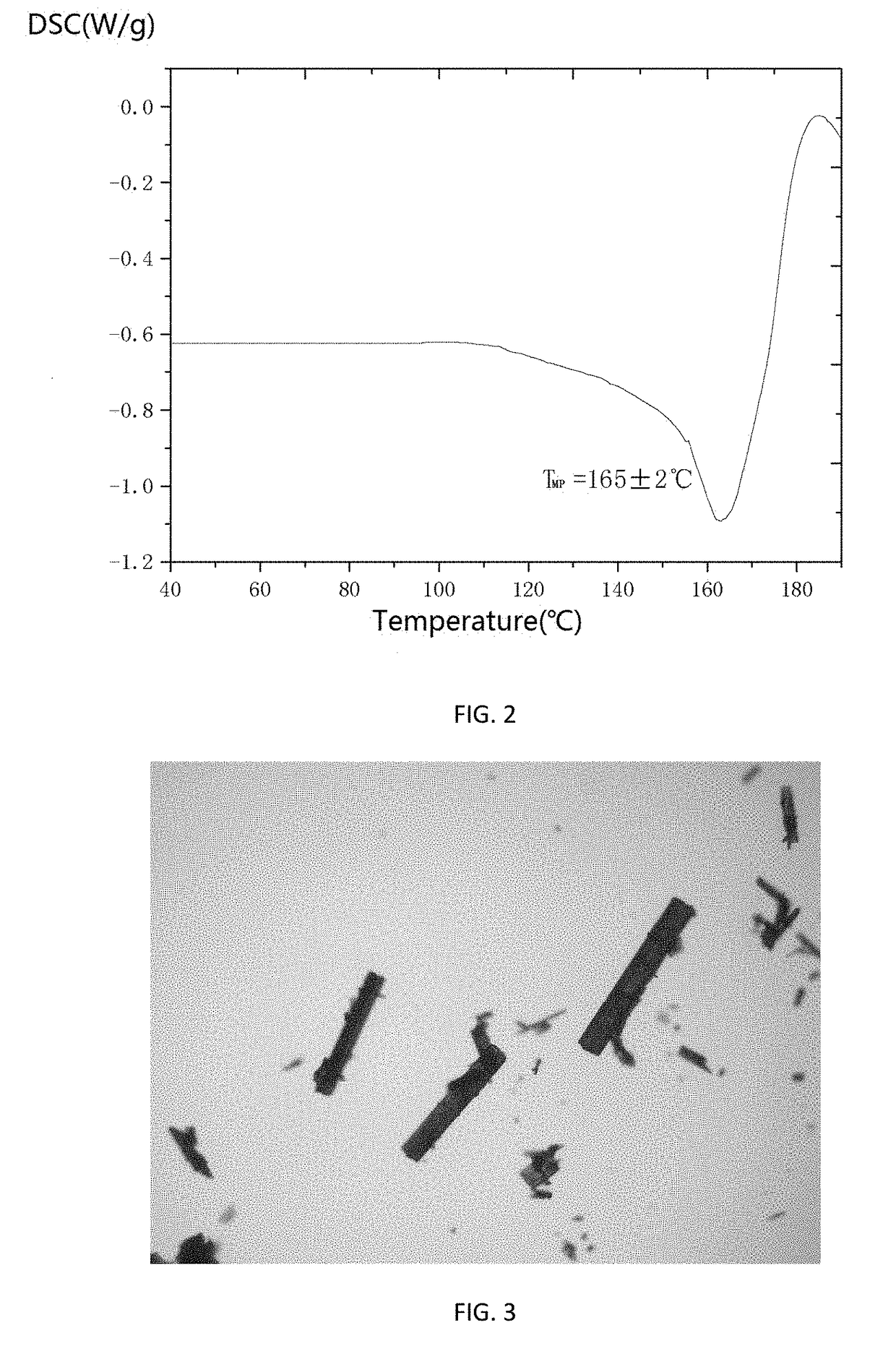
The invention relates to a cefamandole nafate new crystal form and a preparing method thereof. The cefamandole nafate new crystal form is characterized by characterizing the characteristic peaks of a diffraction angle 2 theta degree and a DSC by using X-ray powder diffraction patterns, wherein the new crystal form of the cefamandole nafate compound as V. The preparing method includes the following steps of adding the cefamandole nafate solid into the good solvent at the temperature of 10-30 DEG C, mixing to let the cefamandole nafate dissolve completely, wherein the solution concentration is 0.5 to 1.0 g / ml,; adding the poor solvent, and then reducing the system temperature to 0 to 5 DEG C; standing for cultivating the crystal for 4 to 72 hours; filtering, washing, and drying to obtain a cefamandole nafate new crystal form product. The melting range of the new crystal form is 160 to 180 DEG C, and the peak value is 169+ / -2 DEG C which is higher than the melting points of crystal forms which are reported by all the other patents, thus the product thermal stability is improved. The purity, color, and the form of the new crystal form product are not changed after being stored for 100 days under room temperature and dry conditions, so that long time storage is favorably realized.
Owner:TIANJIN UNIV +1
Novel crystalline form of cefamandole nafate compound, preparation and preparing method thereof
A novel crystalline form is defined by diffraction angle 2θ° of X-ray powder diffraction pattern and characteristic peak of differential scanning calorimetry (DSC). The novel crystalline form of Cefamandole Nafate is prepared as follows: adding Cefamandole Nafate in solid state to an organic solvent to form a suspension with a concentration of 0.04˜0.3 g / ml, stirring the suspension at 40˜50° C. for a period of time, and then cooling to 5˜15° C. at certain cooling rate, continuing to stir for a period of time, then suction filtrating the obtained suspension, the resulting filer cake is Cefamandole Nafate as wet product, which is dried to constant weight to provide the novel crystalline form of Cefamandole Nafate as final product.
Owner:YINING AIERXING INTELLETUAL PROPERTY SERVICE CO LTD
Suspension powder injection of cefamandole nafate and new application thereof
InactiveCN101637456BImprove stabilityEnsure product quality is qualifiedAntibacterial agentsPowder deliveryMedicineSuppurative cholangitis
The invention relates to suspension powder injection of cefamandole nafate and a preparation method thereof and further relates to new application of the injection in treating acute suppurative cholangitis. The frozen-dried suspension powder injection comprises the following components in parts by weight: 1 part of cefamandole nafate, 1.5-12 parts of surfactant and 2-15 parts of frozen-dried supporting agents.
Owner:HAINAN YONGTIAN PHARMA INST
Cefamandole nafate compound entity used for children and preparation thereof
InactiveCN105001240AIncrease internal pressureImprove solubilityPowder deliveryOrganic chemistrySodium bicarbonateFiltration
The invention provides a cefamandole nafate compound entity used for children. The structural formula is as shown in specifications. The cefamandole nafate compound entity is prepared through the following steps that step1, a cefamandole nafate crude product is dissolved in water, sodium hydrogen carbonate is added, pH is adjusted to 6.5-7.5, active carbon stirring and discoloring are performed, and then filtering is performed; step2, an extracting agent is added into filtrate, and then the filtrate is transferred into a pressure-resistant container in a filling mode and taken out after being defoamed and frozen in a temperature control mode; step3, an organic phase is removed, after a solid is melted, ethyl alcohol is dripped, and then slow stirring, crystal growing, filtration washing and vacuum drying are performed to obtain the cefamandole nafate finished product. The cefamandole nafate prepared through the method has the advantages of having few impurities, being high in purity and the like compared with a traditional technology.
Owner:ZHEJIANG CHANGDIAN PHARMA
Cefamandole nafate refining method
The invention provides a cefamandole nafate refining method. The method comprises the steps of A, dissolving polyvinylpyrrolidone in a lower alcohol and water mixed solvent to prepare a polyvinylpyrrolidone solution of 0.1-1.0 mol / L, then adding a cefamandole nafate crude product, increasing temperature to 50-80 DEG C, adding activated carbon after complete dissolution, conducting stirring for 10-30 min, and then conducting filtration to obtain a filtrate for use; B, adding sodium iso-octoate of 15% to the filtration obtained in the step A dropwise, and regulating pH to 6.0-7.0, so that a cefamandole nafate crude product solution is obtained; C, adding acetone to the cefamandole nafate crude product solution obtained in the step B dropwise while stirring, then conducting ultrasonic treatment for 10-30 min, continuing to add acetone, conducting stirring at indoor temperature for 1-2 h to enable a large number of crystals to be separated out, conducting filtration, conducting washing with a small amount of ethyl alcohol, and conducting vacuum drying so that a cefamandole nafate refined product can be obtained, wherein the amount of acetone added at the second time is 0.8-1.2 times that of acetone added at the first time. The refining method has the advantages that yield and purity are high, and the product is high in stability and light in color.
Owner:顾伟
Cefamandole nafate powder injection, production method of powder injection and raw machine thereof
ActiveCN100484516CHigh purityHigh yieldAntibacterial agentsOrganic active ingredientsSodium carbonate anhydrousPowder injection
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com