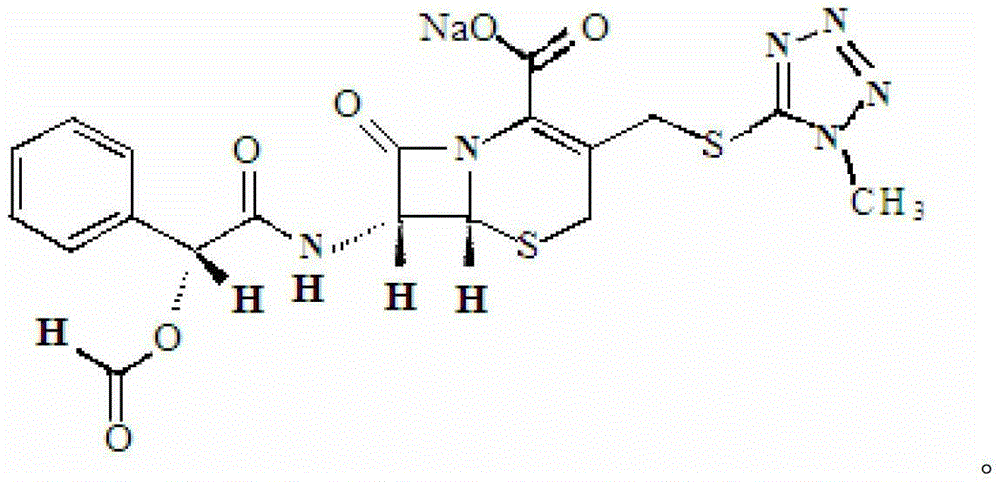
Medicine composite containing cefamandole nafate compound
A cefamandole sodium and compound technology, which is applied in the directions of medical preparations containing active ingredients, organic chemistry, antibacterial drugs, etc., can solve the problems of high equipment requirements, inconvenient use and long time, and achieves good stability, It is not easy to stick to the bottle, and the effect of high fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of cefamandole sodium crystals:
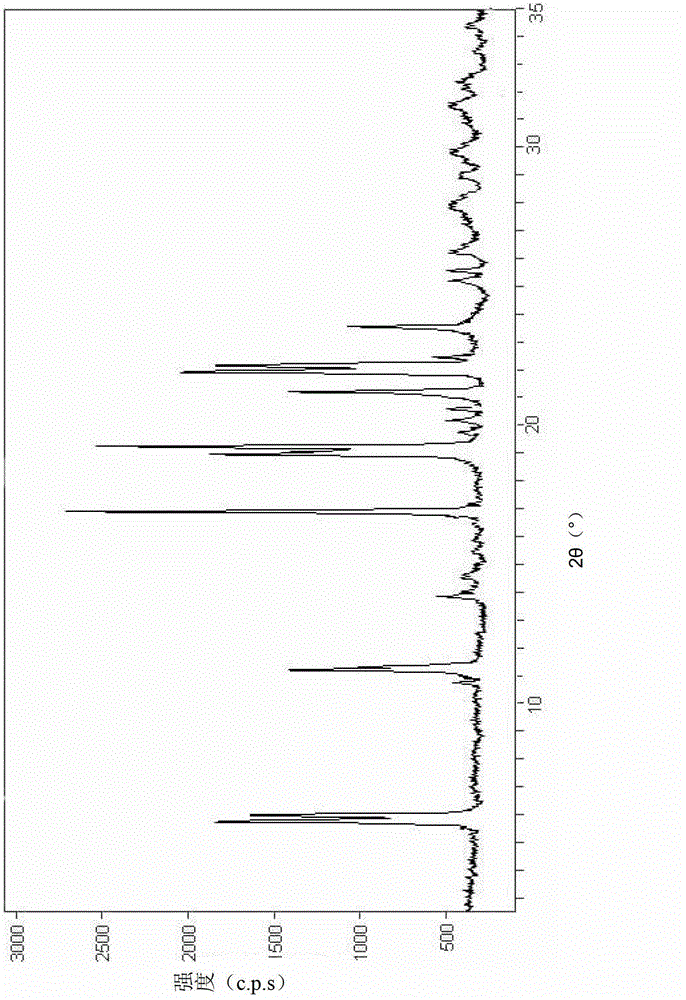
[0026] At a temperature of 35°C, dissolve 3.0g of cefamandole sodium solid in 9.2mL of ethanol and distilled water, wherein the volume ratio of ethanol to distilled water is 1:10, and apply it in the horizontal direction of the liquid surface of the mixed solution A constant magnetic field with a magnetic field strength of 0.6T was left standing at 5°C for 6 hours to obtain white crystals, which were filtered, the filter cake was washed twice with ethanol, and vacuum-dried for 2 hours to obtain cefamandole sodium crystals. The powder XRD diffraction pattern of this cefamandole sodium crystal (as figure 1 shown), at 5.9°, 6.02°, 11.3°, 16.9°, 17.9°, 18.0°, 21.1°, 22.0°, 22.1° and 23.5° diffraction angles (2θ ± 0.01) are shown.
Embodiment 2
[0028] Preparation of cefamandole sodium crystals:
[0029] At a temperature of 40°C, dissolve 3.0g of cefamandole sodium solid in 11.5mL of ethanol and distilled water, wherein the volume ratio of ethanol to distilled water is 1:9, and apply in the horizontal direction of the liquid surface of the mixed solution A constant magnetic field with a magnetic field strength of 1.0 T was left standing at 2° C. for 9 hours to obtain white crystals, which were filtered, the filter cake was washed twice with ethanol, and vacuum-dried for 2 hours to obtain cefamandole sodium crystals. Show through powder XRD detector analysis, accord with the result shown in accompanying drawing.
Embodiment 3
[0031] Preparation of cefamandole sodium crystals:
[0032] At a temperature of 30°C, dissolve 3.0g of cefamandole sodium solid in 14.8mL of ethanol and distilled water, wherein the volume ratio of ethanol to distilled water is 1:10, and apply it in the horizontal direction of the liquid surface of the mixed solution A constant magnetic field with a magnetic field strength of 0.5 T was left standing at 0° C. for 7 hours to obtain white crystals, which were filtered, the filter cake was washed twice with ethanol, and vacuum-dried for 2 hours to obtain cefamandole sodium crystals. Show through powder XRD detector analysis, accord with the result shown in accompanying drawing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com