Method of synthesizing antibiotics cefamandole nafate
A technology of cefamandide sodium and a synthesis method, which is applied in directions such as organic chemistry, can solve problems such as affecting acylation reaction, increase manufacturing cost, increase operation steps, etc., and achieves simple process operation, good product quality and product yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
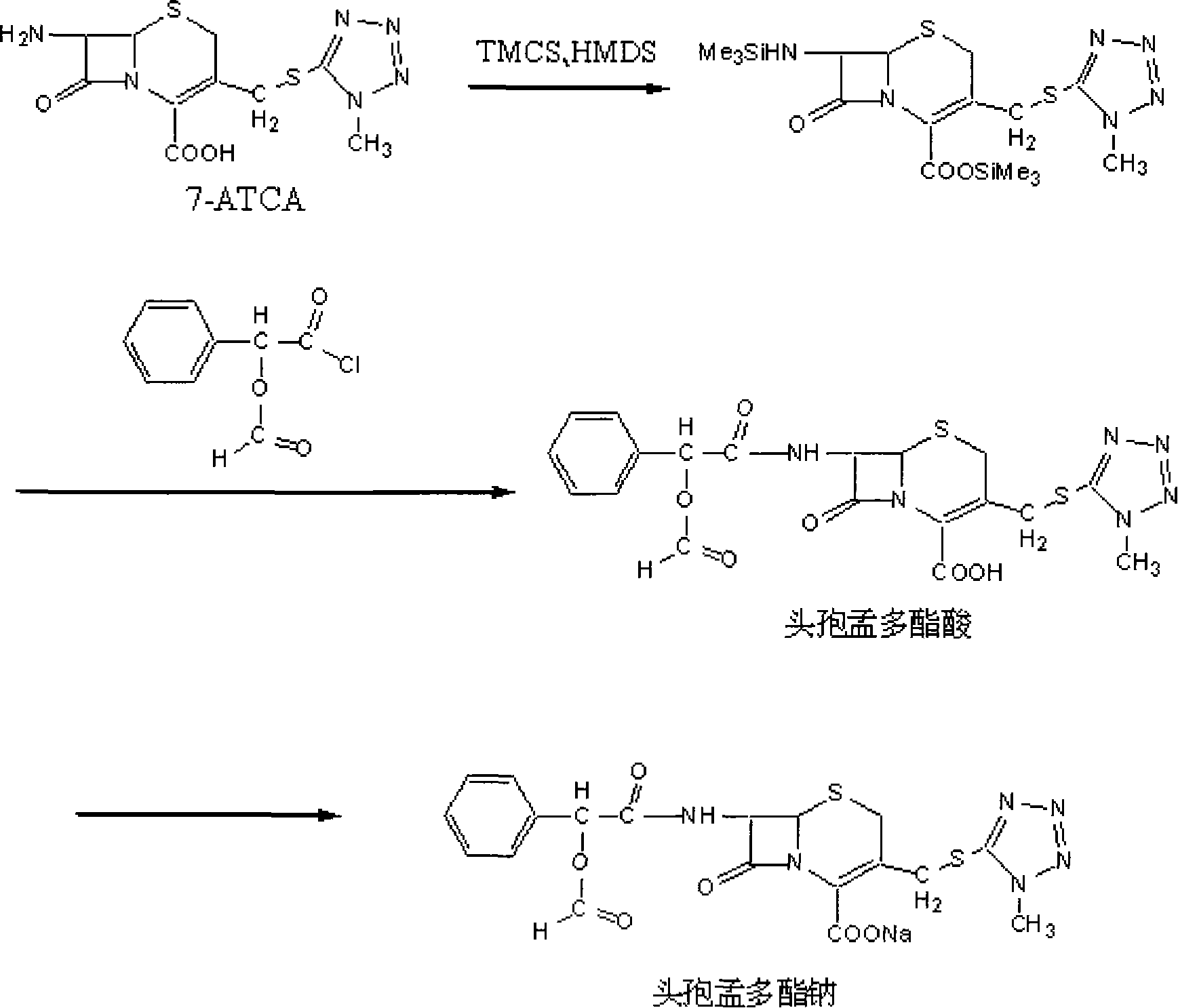
[0025] In a 500ml four-necked reaction flask, add 300ml ethyl acetate, 30g 7-ATCA (0.092mol), control the temperature at 20~25℃, add 13.5ml HMDS (0.064mol), add 8.2ml dropwise while stirring Trimethylchlorosilane (0.064mol). After the dropwise addition is completed, the temperature is raised to 35-40°C for reaction, and after 3 to 4 hours of reaction, the temperature is lowered to 25-30°C to obtain a silicon ester solution of 7-ATCA.
[0026] Add 15.6ml D-(-)-2-formyloxy-2-phenylacetyl chloride (0.092mol) dropwise to the above-mentioned 7-ATCA silicon ester solution. After 30 minutes of dropping, keep the temperature at 25 At -30°C, the reaction was carried out with stirring for 1.5 to 2 hours, and the end of the reaction was detected by HPLC (the concentration of remaining 7-ATCA in the reaction solution was less than 1 mg / ml).
[0027] 200ml of deionized water was added to carry out the hydrolysis reaction, stirred for 20 minutes, the water layer was removed, the organic layer c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com