Method for preparing cefamandole nafate powder injection preparation
A technology of sodium montoroleate powder and cephalosporin, applied in the field of medicine, can solve the problems of long reaction time and high content of impurities, and achieve the effects of short reaction time, low content of impurities and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
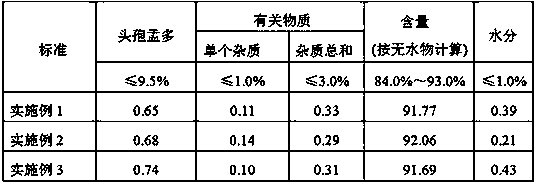
Embodiment 1
[0026] Add 75mL of dichloromethane, 25g of 7-ACT, and 28.8g of BSA into a 500mL four-neck flask, raise the temperature to 35°C, react for 30min, dissolve, and cool down after the reaction. Control the temperature at -5-0°C, add 15 mL of formylmandelic acid chloride dropwise, react for 5 minutes after the addition is complete, and detect that the 7-ACT residue is <2.0%, and the reaction is over. Add 50mL of purified water to another four-necked bottle, keep the temperature below 15°C, feed the reaction liquid into the four-necked bottle, and at the same time add 15wt% sodium hydroxide solution, control the pH=4.8±0.1, stir rapidly for 5min , stand still for phase separation, and recover the organic phase. Add 150 mL of ethyl acetate to the water phase, adjust the pH to 1.25 with 6 mol / L hydrochloric acid solution, stir rapidly for 10 min, let stand to separate the phases, and discard the water phase.
[0027] Add 2.5g of activated carbon and 2.5g of anhydrous magnesium sulfate...
Embodiment 2
[0030] Add 150mL of dichloromethane, 25g of 7-ACT, 20.6g of BSA, and 5.5g of trimethylchlorosilane into a 500mL four-neck flask, heat up to 42°C, react for 50min, dissolve and clear, and cool down after the reaction is complete. Control the temperature at -10~-5°C, add 15 mL of formylmandelic acid chloride dropwise, and react for 20 minutes after the addition is complete. The 7-ACT residual is detected to be <2.0%, and the reaction is over. Add 50mL of purified water to another four-necked flask, keep the temperature below 15°C, feed the reaction liquid into the four-necked flask, and at the same time add 10wt% sodium bicarbonate solution, control the pH=5.5±0.1, stir rapidly for 5min , stand still for phase separation, and recover the organic phase. Add 150 mL of ethyl acetate to the water phase, adjust the pH to 0.52 with 6 mol / L hydrochloric acid solution, stir rapidly for 10 min, let stand to separate the phases, and discard the water phase.
[0031] Add 2.5g of activated...
Embodiment 3
[0034] Add 125mL of dichloromethane, 25g of 7-ACT, and 30.5g of BSA into a 500mL four-neck flask, heat up to 30°C, react for 60min, dissolve and clear, and cool down after the reaction is complete. Control the temperature at -10~-5°C, add 15 mL of formylmandelic acid chloride dropwise, and react for 10 minutes after the addition is complete. The 7-ACT residual is detected to be <2.0%, and the reaction is over. Add 50mL of purified water into another four-necked flask, keep the temperature below 15°C, feed the reaction liquid into the four-necked flask, and at the same time add 10wt% sodium carbonate solution, control the pH=6.5±0.1, stir rapidly for 5min, Stand to separate the phases and recover the organic phase. Add 150 mL of ethyl acetate to the water phase, adjust the pH to 1.35 with 6 mol / L hydrochloric acid solution, stir rapidly for 10 min, let stand to separate the phases, and discard the water phase.
[0035] Add 2.5g of activated carbon and 2.5g of anhydrous magnesi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com