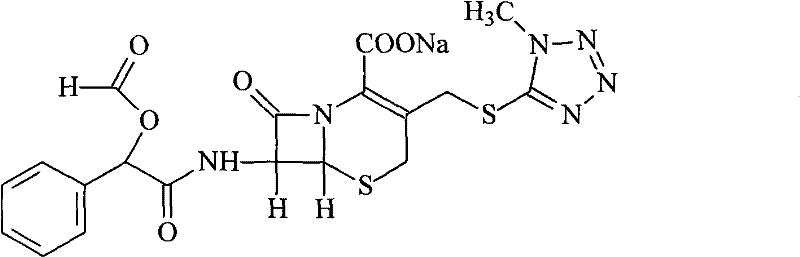
Suspension powder injection of cefamandole nafate and new application thereof
A technology of cefamandole sodium and spamandole sodium, which is applied in the direction of powder transportation, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of poor stability and stability of cefamandole sodium low cost, high bioavailability, and avoid phase separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
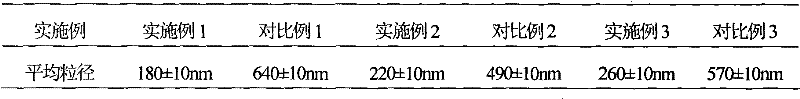
Embodiment 1
[0034] The preparation of embodiment 1 cefamandole sodium suspension powder injection
[0035] Prescription (100 bottles):
[0036] Cefamandole Sodium 50g
[0037] Cholesterol 83.3g
[0038] Poloxamer 188 41.7g
[0039]Mannitol 75g
[0040] Sorbitol 75g
[0041] Preparation Process
[0042] (1) Add 83.3g of cholesterol and 41.7g of poloxamer 188 into 1000ml of water for injection, then add 50g of cefamandole sodium and mix evenly, heat and stir in a water bath at 80°C until it melts;
[0043] (2) Heat the above liquid at 70-90°C and use a tissue masher to shear and stir for 15 minutes at a speed of 12000r / min to obtain a primary emulsion, and then circulate and emulsify it through a high-pressure homogenizer for 5 times to obtain an emulsion;
[0044] (3) Add 75g of mannitol and 75g of sorbitol into the emulsion, filter and subpackage after dissolving, and freeze-dry to obtain cefamandole sodium suspension powder injection.
Embodiment 2
[0053] The preparation of embodiment 2 cefamandole sodium suspension powder injection
[0054] Prescription (100 bottles):
[0055] Cefamandole Sodium 100g
[0056] Cholesterol 333.3g
[0057] Poloxamer 188 166.7g
[0058] Glucose 175g
[0059] Glycine 525g
[0060] Preparation Process
[0061] (1) Add 333.3g of cholesterol and 166.7g of poloxamer 188 into 6500ml of water for injection, then add 100g of cefamandole sodium and mix evenly, heat and stir in a water bath at 70°C until it melts;
[0062] (2) Keeping the above liquid at 70-90°C for 20 minutes with a tissue masher to shear and stir at a speed of 10,000 r / min to obtain a primary emulsion, and then circulate and emulsify it through a high-pressure homogenizer for 4 times to obtain an emulsion;
[0063] (3) Add 175g of glucose and 525g of glycine to the emulsion, filter and subpackage after dissolving, and freeze-dry to obtain cefamandole sodium suspension powder injection.
Embodiment 3
[0072] The preparation of embodiment 3 cefamandole sodium suspension powder injection
[0073] Prescription (100 bottles):
[0074] Cefamandole Sodium 200g
[0075] Cholesterol 600g
[0076] Poloxamer 188 300g
[0077] Trehalose 100g
[0078] Lactose 500g
[0079] Preparation Process
[0080] (1) Add 600g of cholesterol and 300g of poloxamer 188 into 7000ml of water for injection, then add 200g of cefamandole sodium and mix evenly, heat and stir in a water bath at 90°C until molten;
[0081] (2) Keeping the above liquid at 70-90°C for 20 minutes with a tissue masher to shear and stir at a speed of 15000r / min to obtain a primary emulsion, and then circulate and emulsify it through a high-pressure homogenizer for 5 times to obtain an emulsion;
[0082] (3) Add 100g trehalose and 500g lactose to the emulsion, dissolve, filter, subpackage, and freeze-dry to obtain cefamandole sodium suspension powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com