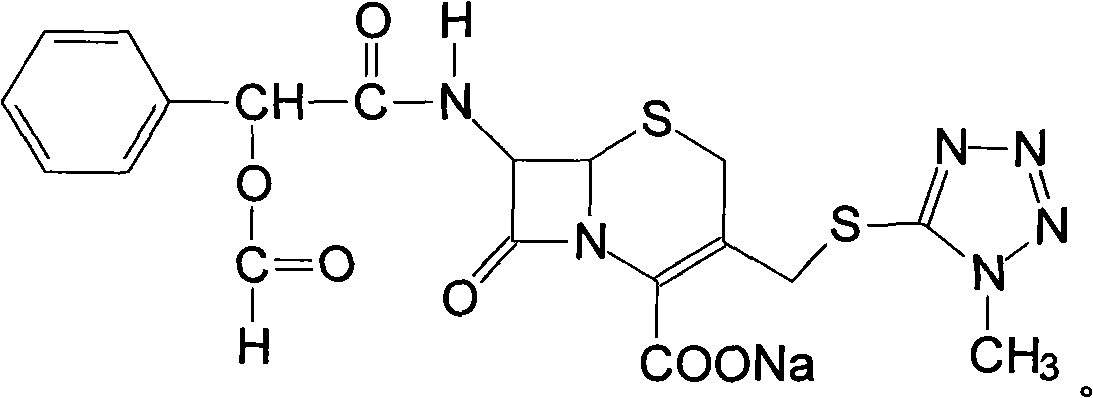
Preparation method of cefamandole nafate
A technology for cefmandole sodium and cefene, which is applied in the field of preparation of cefmandole sodium, can solve the problems of complicated process operation, influence on product yield and final product quality, etc., and achieves simple operation process, low cost and simple cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
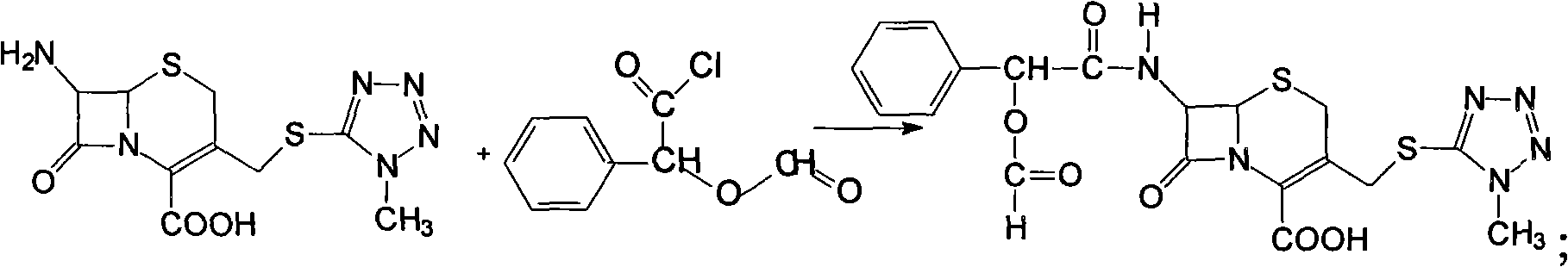
[0035] Preparation of 7-D-(2-formyloxyphenylacetamide)-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-3-cephem-4-carboxylic acid
[0036]Suspend 258g (0.798mol) of 7-amino-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-3-cephem-4-carboxylic acid and 375g of sodium bicarbonate in Add 1000ml of acetone solution containing 148.8g (0.75mol) of α-formylmandelic acid chloride to a mixture of 7500ml of water and 7500mL of acetone that has been precooled to 5°C, keep stirring at 5°C for 1 hour, then heat up Continue to stir and react at 20°C for 2 hours. After the reaction is over, distill off the acetone at 20°C under reduced pressure, add ethyl acetate to the raffinate for extraction, and extract twice. The amount of ethyl acetate each time is 6L, and the two extractions are combined. liquid, dried over anhydrous sodium sulfate, concentrated under reduced pressure at 20°C to dryness to obtain a light yellow oil; recrystallized the light yellow oil with ether to obtain 383 g of a light yellow ...
Embodiment 2
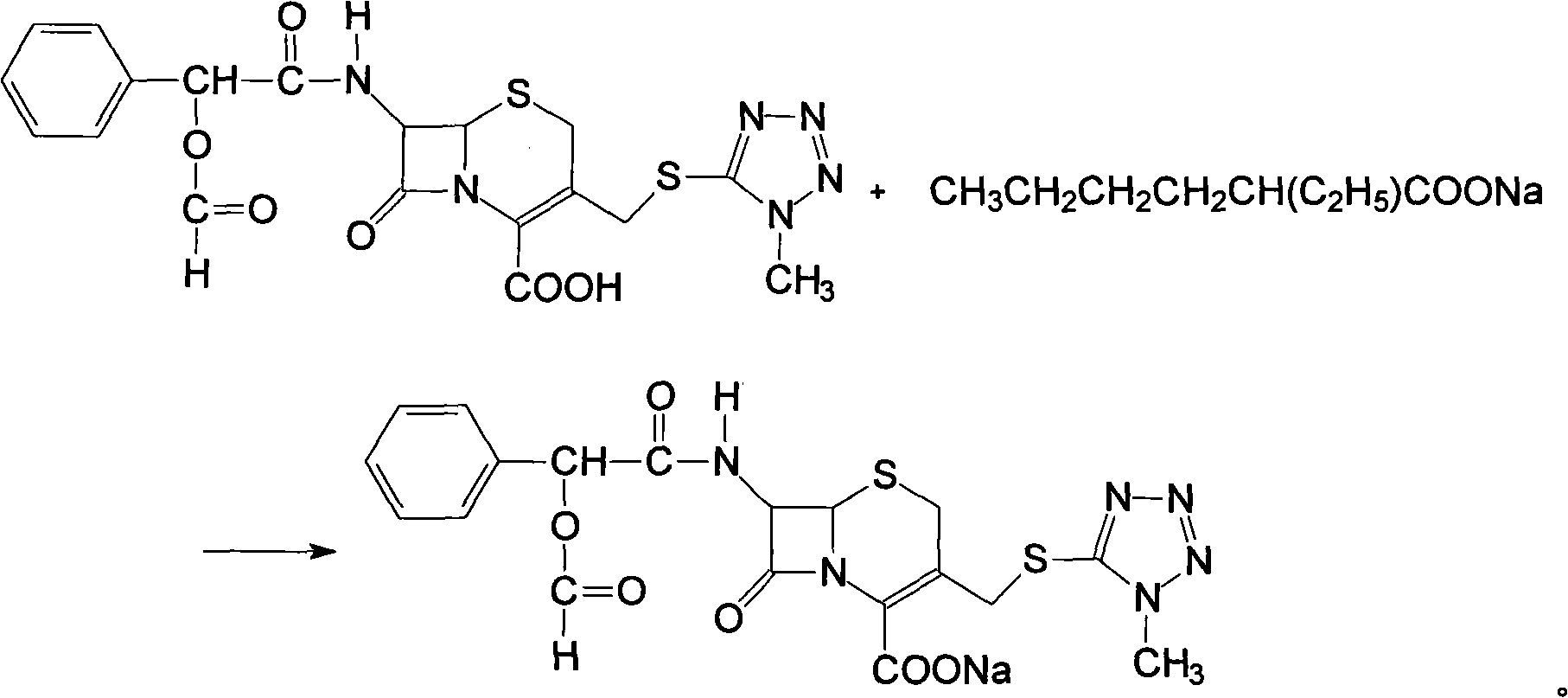
[0040] 7-D-(2-formyloxyphenylacetamide)-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-3-cephem-4-carboxylic acid sodium salt preparation
[0041] Dissolve 383 g of the light yellow powder obtained in Example 1 in 1.5 L of acetone, then add 1.2 L of acetone solution containing 92 g (0.55 mol) of sodium isooctanoate, cool to 0° C. in an ice bath, continue stirring for 3 hours, filter, The filter cake was washed with acetone and dried under reduced pressure at 40°C for 5 hours to obtain 276.2 g of crude product with a melting point (mp) of 183°C-185°C. The molar yield was 93.8%.
[0042] The above-mentioned crude product is carried out elemental analysis, the result is as follows: C: 44.7%, H: 3.5%, N: 16.3%, O: 18.6%, S: 12.5%, Na: 4.4% (all are mass percent), and 7- Theoretical value of D-(2-formyloxyphenylacetamide)-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-3-cephem-4-carboxylic acid sodium salt : C: 44.5%, H: 3.4%, N: 16.4%, O: 18.7%, S: 12.5%, Na: 4.5% (both mass percentages)...
Embodiment 3
[0045] Preparation of Refined Product of Cefamandole Sodium
[0046] The 276.2g crude product obtained in Example 2 was dissolved in 5000ml of anhydrous methanol, 10g of gac was added under stirring, stirred and decolorized for 30 minutes, filtered to obtain the filtrate, the filtrate was slowly added to 15000ml of ethyl acetate, at room temperature Continue to stir and crystallize for 4 hours, filter, wash the filter cake with a mixed solution of anhydrous methanol and ethyl acetate (the volume ratio of anhydrous methanol to ethyl acetate is 1:1), collect the filter cake, and dry under vacuum at 40°C to obtain The refined product of cefamandole sodium is 256.3g, the yield is 92.8%, and the mp of the refined product of cefamandole sodium is 189°C-190.5°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com