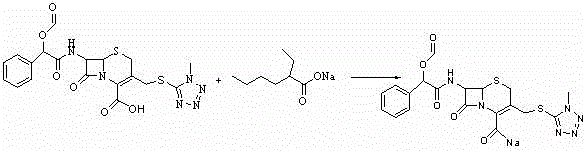
Synthesis method for dextrorotation cefamandole nafate
A technology of cefamandole sodium and its synthetic method, which is applied in the direction of organic chemistry, can solve problems such as difficult methods, complicated treatment, high toxicity, etc., and achieve the effects of easy-to-obtain raw materials, simple operation methods, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of 7-ATCA
[0041] Add 43.5g of acetonitrile, 7.1g of mercaptotetrazolium, 16g of 7-ACA into the four-neck flask, stir evenly, and add BF within 15 minutes 3 - 63g of acetonitrile, raise the inner temperature to 35~39°C, react with stirring for 50~60 minutes, and cool to the inner temperature of 28~32°C. Add (14.76g hydrochloric acid + 12.6g water) mixed solution within 15 minutes, keep the temperature, stir for 40 minutes, cool down to 10~20°C, and grow the crystal for 1 hour. Suction filtration, top washing with 120ml of acetone in 4 times. Vacuum dry at 45°C for 15 hours. (Moisture content ≤ 1.0% before unloading) 19.5 g of solid was obtained, with a molar yield of 95.1% and a purity of 99.9%.
Embodiment 2
[0042] Example 2 Preparation of 7-ATCA
[0043] Add 43.5g of dimethyl carbonate, 7.1g of mercaptotetrazolium, 16g of 7-ACA into the four-necked bottle, stir well, and add BF within 15 minutes 3 - Dimethyl carbonate 63g, raise the inner temperature to 35~39°C, react under stirring for 50~60 minutes, then cool to the inner temperature of 28~32°C. Add (14.76g hydrochloric acid + 12.6g water) mixed solution within 15 minutes, keep the temperature, stir for 40 minutes, cool down to 10~20°C, and grow the crystal for 1 hour. Suction filtration, top washing with 120ml of acetone in 4 times. Vacuum dry at 45°C for 15 hours. 19.5 g of solid was obtained, the molar yield was 95.1%, and the purity was 99.9%.
Embodiment 3
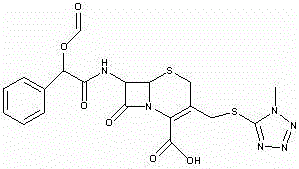
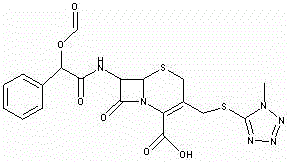
[0044] Example 3 Preparation of Dextrocefamandol Sodium
[0045] Add 175ml of ethyl acetate into the four-necked bottle, add 19g of the first-step product 7-ATCA under stirring, add 8.76g of trimethylchlorosilane and 11.37g of hexamethyldisilazane, raise the internal temperature to 54~56°C, and react 80~90 minutes. After the reaction, cool down to 28~32°C, add 5.6g of acetamide, continue cooling down to -5~-10°C, add 11.83g of S-(-)-formylated mandelic acid chloride (internal temperature ≤ 2°C). Maintain the internal temperature at -2~-7°C for 15~30 minutes, add 115ml of water at 2~4°C (internal temperature ≤4°C), maintain the internal temperature for 15~25 minutes, raise the temperature to 10°C, separate the phases, and discard the water phase , and the ester phase was transferred to another four-necked bottle. Add 350ml of isopropanol and raise the temperature to 20~25°C.
[0046] Add the prepared acetone / sodium isooctanoate solution (104g / 10g) into the four-necked bottle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com